Mammalian tRNA acetylation determines translation efficiency and tRNA quality control
- PMID: 40595590
- PMCID: PMC12215905
- DOI: 10.1038/s41467-025-60723-3
Mammalian tRNA acetylation determines translation efficiency and tRNA quality control
Abstract
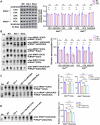
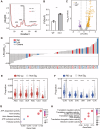
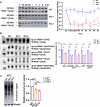
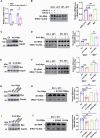
Acetylation is a conserved and pivotal RNA modification. Acetylation of tRNA occurs at C12 (ac4C12) in eukaryotic tRNAs. Yeast ac4C12 prevents tRNASer from rapid tRNA decay (RTD) at higher temperatures. However, the biological function of ac4C12 in higher eukaryotes remains unexplored. Moreover, whether mammalian cells contain an RTD pathway is unclear. Here, we deleted Thumpd1, the indispensable factor for ac4C12 biogenesis, in NIH/3T3 cells. Loss of ac4C12 significantly reduced tRNA aminoacylation and translational efficiency physiologically, in particular, of those enriched with Ser/Leu codons with two U/A nucleotides. Remarkably, ac4C12 hypomodification selectively generated rapid tRNALeu(CAG) turnover under heat stress. We demonstrated that tRNALeu(CAG) was degraded by a mammalian RTD (mRTD) mechanism, consisting of Xrn1/Xrn2-mediated 5'-3' exonuclease digestion and intracellular pAp level control by Bpnt1/Bpnt2. Our results reveal both the pivotal roles of ac4C12 in translation and a mRTD pathway for tRNA quality control under heat stress in mammalian cells.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Photosynthetic demands on translational machinery drive retention of redundant tRNA metabolism in plant organelles.Proc Natl Acad Sci U S A. 2024 Dec 24;121(52):e2421485121. doi: 10.1073/pnas.2421485121. Epub 2024 Dec 18. Proc Natl Acad Sci U S A. 2024. PMID: 39693336 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Nanopore sequencing of intact aminoacylated tRNAs.Nat Commun. 2025 Aug 20;16(1):7781. doi: 10.1038/s41467-025-62545-9. Nat Commun. 2025. PMID: 40835813 Free PMC article.
-
tRNA modifications tune m6A-dependent mRNA decay.Cell. 2025 Jul 10;188(14):3715-3727.e13. doi: 10.1016/j.cell.2025.04.013. Epub 2025 Apr 30. Cell. 2025. PMID: 40311619
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

