Controlled assembly of rotaxane translational isomers using dual molecular pumps
- PMID: 40595599
- PMCID: PMC12216197
- DOI: 10.1038/s41467-025-61364-2
Controlled assembly of rotaxane translational isomers using dual molecular pumps
Abstract
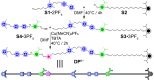
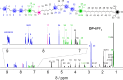
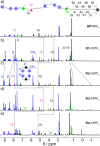
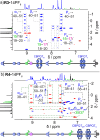
The ability to control the relative motion between different components of molecules with precision is a cornerstone of synthetic nanotechnology. Mechanically interlocked molecules such as rotaxanes offer a platform for exploring this control by means of the positional manipulation of their components. Here, we demonstrate the use of a molecular dual pump to achieve the assembly of translational isomers with high efficiency and accuracy. By harnessing pumping cycles, rings can be guided selectively along a molecular axle, resulting in two sets of distinct translational isomers of [2]- and [3]rotaxanes. These isomers, produced in high yields, are characterized by mass spectrometry in addition to one- and two-dimensional nuclear magnetic resonance spectroscopy, which collectively reveal the location of the rings in the rotaxanes. Nuclear Overhauser effect spectroscopy confirms the spatial localization of rings, while diffusion ordered spectroscopy measurements quantifies the differences in hydrodynamic properties between the rotaxanes. This research supports the status of molecular pumps as a robust tool for precise nanoscale assembly, while advancing the practice of molecular machinery at the frontiers of synthetic nanotechnology.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Eliel, E. L. Stereochemistry of Organic Compounds (Wiley, 1994).
-
- Leigh, J. Principles of Chemical Nomenclature A Guide to IUPAC Recommendations2011Edition:A. Guide to IUPAC Recommendations2011 Edition (The Royal Society of Chemistry, 2011).
-
- Favre, H. A. & Powell, W. H. Nomenclature of Organic Chemistry (The Royal Society of Chemistry, 2013).
-
- Oki, M. Reactivity of conformational isomers. Acc. Chem. Res.17, 154–159 (1984). - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

