Single cell transcriptomics of human kidney organoid endothelium reveals vessel growth processes and arterial maturation upon transplantation
- PMID: 40595660
- PMCID: PMC12216864
- DOI: 10.1038/s41536-025-00418-x
Single cell transcriptomics of human kidney organoid endothelium reveals vessel growth processes and arterial maturation upon transplantation
Abstract
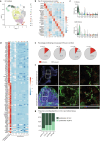
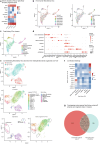
Kidney organoids derived from human induced pluripotent stem cells lack a proper vasculature, hampering their applicability. Transplantation prevents the loss of organoid endothelial cells (ECs) observed in vitro, and promotes vascularization. In this study, we transplanted kidney organoids in chicken embryos and deployed single-cell RNA sequencing of ~12,000 organoid ECs to delineate their molecular landscape and identify key changes associated with transplantation. Transplantation significantly altered EC phenotypic composition. Consistent with angiogenesis, proliferating EC populations expanded 8 days after transplantation. Importantly, ECs underwent a major vein-to-arterial phenotypic shift. One of the transplantation-specific arterial EC populations, characterized by laminar shear stress response and Notch signalling, showed a similar transcriptome as human fetal kidney arterial/afferent arteriolar ECs. Consistently, transplantation-induced transcriptional changes involved proangiogenic and arteriogenic SOX7 transcription factor upregulation and regulon enrichment. These findings point to blood flow and candidate transcription factors such as SOX7 as possible targets to enhance kidney organoid vascularization.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
3D organoids containing endothelial and neural cells generation by serial inductions of differentiation on human iPSC-derived embryoid bodies.bioRxiv [Preprint]. 2025 May 21:2025.05.20.653559. doi: 10.1101/2025.05.20.653559. bioRxiv. 2025. PMID: 40475416 Free PMC article. Preprint.
-
Unlocking the full potential of human pluripotent stem cell-derived kidney organoids through bioengineering.Kidney Int. 2025 Jul;108(1):38-47. doi: 10.1016/j.kint.2025.01.043. Epub 2025 Apr 23. Kidney Int. 2025. PMID: 40280411 Review.
-
Ethical considerations for advancing research using organoid models derived from the placenta.Hum Reprod Update. 2025 Jul 1;31(4):392-401. doi: 10.1093/humupd/dmaf007. Hum Reprod Update. 2025. PMID: 40096642 Free PMC article. Review.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD010216. doi: 10.1002/14651858.CD010216.pub7. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 Jan 8;1:CD010216. doi: 10.1002/14651858.CD010216.pub8. PMID: 36384212 Free PMC article. Updated.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2021 Sep 14;9(9):CD010216. doi: 10.1002/14651858.CD010216.pub6. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Nov 17;11:CD010216. doi: 10.1002/14651858.CD010216.pub7. PMID: 34519354 Free PMC article. Updated.
References
Grants and funding
- NNF21CC0073729/Novo Nordisk Foundation
- NNF21OC0071718/Novo Nordisk Foundation
- NNF21OC0072031/Novo Nordisk Foundation
- NNF21CC0073729/Novo Nordisk Foundation
- NNF21CC0073729/Novo Nordisk Foundation
- ANR24-CPJ1-0181-01/Agence Nationale de la Recherche
- FWO - 11A1520N|11A1522N/Fonds voor Wetenschappelijk Onderzoek
- R396-2022-350/Lundbeck Foundation
- METH.14.08/Long-term structural Methusalem funding from the Flemish government
- EU-101055155/European Research Council ERC Advanced Research Grant
- FWO-Vlaanderen/Fund for Scientific Research-Flanders
LinkOut - more resources
Full Text Sources

