State- and time-resolved observation of ultrafast intermolecular proton transfer in hydrated biomolecules
- PMID: 40595688
- PMCID: PMC12214634
- DOI: 10.1038/s41467-025-61305-z
State- and time-resolved observation of ultrafast intermolecular proton transfer in hydrated biomolecules
Abstract
Proton transfer underpins number of chemical and biochemical processes, yet its sub-100 fs dynamics have rarely been captured in real time. Here, we report direct and time-resolved observation of ionizing radiation-induced proton transfer in a heteroaromatic hydrate: the pyrrole-water complex. Both the electron-impact and strong-field laser experiments create a locally and doubly charged pyrrole unit (C4H5N2+), which immediately (within 60 fs) donates a proton to the adjacent H2O, generating deprotonated C4H4N+ and hydronium H3O+ cations that subsequently undergo Coulomb explosion. The electron-impact experiments directly revealed initial states and provided dynamical insights through fragment ions and electron coincidence momentum imaging. The strong-field femtosecond laser experiments tracked the ultrafast dynamics of proton transfer; complementary ab initio calculations unraveled the dynamical details. The 50-60 fs proton transfer qualifies as one of the fastest acid-base reactions observed to date. This study offers a novel perspective on radiation-induced proton transfer in hydrated biomolecules.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
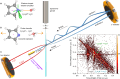
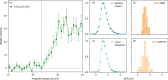
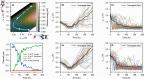
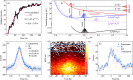
References
-
- Mohammed, O. F., Pines, D., Dreyer, J., Pines, E. & Nibbering, E. T. J. Sequential proton transfer through water bridges in acid-base reactions. Science310, 83–86 (2005). - PubMed
-
- Nocera, D. G. Proton-coupled electron transfer: The engine of energy conversion and storage. J. Am. Chem. Soc.144, 1069–1081 (2022). - PubMed
-
- Hvasanov, D., Peterson, J. R. & Thordarson, P. Self-assembled light-driven photosynthetic-respiratory electron transport chain hybrid proton pump. Chem. Sci.4, 3833–3838 (2013).
-
- Wikström, M., Sharma, V., Kaila, V. R. I., Hosler, J. P. & Hummer, G. New perspectives on proton pumping in cellular respiration. Chem. Rev.115, 2196–2221 (2015). - PubMed
-
- Eigen, M. Immeasurably Fast Reactions. Nobel Lectures, Chemistry 1963-1970 (Elsevier Publishing Company, Amsterdam, 1972) https://www.nobelprize.org/prizes/chemistry/1967/eigen/lecture/.
Grants and funding
LinkOut - more resources
Full Text Sources

