Microfluidics unveils role of gravity and shear stress on Pseudomonas fluorescens motility and biofilm growth
- PMID: 40595697
- PMCID: PMC12216817
- DOI: 10.1038/s41522-025-00744-4
Microfluidics unveils role of gravity and shear stress on Pseudomonas fluorescens motility and biofilm growth
Abstract
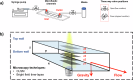
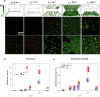
Biofilm proliferation in confined environments is a challenge in biomedical, industrial, and space applications. Surfaces in contact with fluids experience varying bulk stresses due to flow and gravity, factors often overlooked in biofilm studies. This research quantifies the combined effect of gravity and shear stress on Pseudomonas fluorescens SBW25 motility and biofilm growth. Using a rectangular-section microfluidic channel under laminar flow, we compared top and bottom surfaces, where gravity either pulls bacteria away or pushes them toward the surface. Results revealed an asymmetric bacterial distribution, leading to varying surface cell densities and contamination levels. We also analyzed spatial reorganization over time and classified bacterial motility under flow. Findings show that external mechanical stresses influence both motility and biofilm morphology, impacting biocontamination patterns based on shear stress and gravity direction. This study provides insights into biofilm control strategies in diverse environments.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Hall-Stoodley, L., Costerton, J. W. & Stoodley, P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol.2, 95–108 (2004). - PubMed
-
- Azeredo, J. et al. Critical review on biofilm methods. Crit. Rev. Microbiol.43, 313–351 (2017). - PubMed
-
- Høiby, N. et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect.21, S1–S25 (2015). - PubMed
-
- Novikova, N. D. Review of the knowledge of microbial contamination of the Russian manned spacecraft. Microb. Ecol.47, 127–132 (2004). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

