Bioequivalence study of two formulations of lurasidone film coated tablets in healthy subjects under fed conditions
- PMID: 40595770
- PMCID: PMC12214960
- DOI: 10.1038/s41598-025-04800-z
Bioequivalence study of two formulations of lurasidone film coated tablets in healthy subjects under fed conditions
Abstract
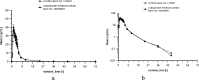
To define the bioequivalence (BE) of two orally administered film coated tablets containing 40 mg lurasidone hydrochloride. A single-dose, open-label, randomized two-way crossover trial was conducted under fed conditions, with a washout period of at least one week. Blood samples were drawn over a period of 72 h and plasma concentrations were analysed using a Liquid Chromatography tandem Mass Spectrometry (LC-MS/MS) method. The Pharmacokinetic (PK) parameters were calculated using Phoenix WinNonlin software, based on non-compartmental analysis. The two one-sided hypothesis at the α = 0.05 level of significance was tested by constructing the 90% confidence interval of two one-sided t-tests for the geometric mean ratios test versus reference preparation with analysis of variance (ANOVA). After oral administration of 40 mg lurasidone hydrochloride under fed conditions, the mean area under the curve (AUCt, AUC∞) and maximum plasma concentrations (Cmax) were 175 ng/mL*h; 184 ng/mL*h and 60 ng/mL, respectively for the test product and 185 ng/mL*h; 198 ng/mL*h and 59 ng/mL respectively for the reference product. 90% Confidence Intervals (CI) for all PK parameters were within the acceptance range of 80.00-125.00%. BE was demonstrated between the generic product and the innovator product in healthy Caucasian male subjects. No clinically meaningful differences in safety or tolerability were observed.
Keywords: Bioequivalence; Lurasidone; Pharmacokinetics; Variation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: All study partners were paid by Sanovel İlac Sanayi ve Ticaret A.S. – Türkiye for their efforts on realising the study and the preparation of the manuscript, on a company basis. No author was personally paid. Gökçe Sözer, Muradiye Nacak, Erol Durucu, Aydın Erenmemişoğlu, Wolfgang Martin, Ewald Ottmann, Martin Reinsch, Selma Alime Koru declare that they have no more conflicts of interest.
Figures

Similar articles
-
Bioequivalence and Tolerability of 2 Lurasidone Formulations: A Randomized, Single-Dose, 2-Period, Crossover Study in Healthy Chinese Subjects Under Fasting and Fed Conditions.Clin Pharmacol Drug Dev. 2025 Jun;14(6):436-442. doi: 10.1002/cpdd.1529. Epub 2025 Apr 28. Clin Pharmacol Drug Dev. 2025. PMID: 40293257 Clinical Trial.
-
Randomized single-dose crossover comparative bioavailability study of two novel oral cannabidiol (CBD) formulations in healthy volunteers under fed conditions, compared to a standard CBD isolate capsule.J Cannabis Res. 2025 Aug 6;7(1):54. doi: 10.1186/s42238-025-00312-9. J Cannabis Res. 2025. PMID: 40770387 Free PMC article.
-
Pharmacokinetics and Safety with Bioequivalence of Isosorbide Mononitrate Sustained-Release Tablets in Chinese Healthy Volunteers: Bioequivalence Study.Drug Des Devel Ther. 2025 Jul 14;19:6025-6035. doi: 10.2147/DDDT.S508202. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40687901 Free PMC article. Clinical Trial.
-
Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis.CNS Drugs. 2012 Sep 1;26(9):733-59. doi: 10.2165/11634500-000000000-00000. CNS Drugs. 2012. PMID: 22900950
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
References
-
- Kanchanatawan, B., Thika, S., Anderson, G., Galecki, P. & Maes, M. Affective symptoms in schizophrenia are strongly associated with neurocognitive deficits indicating disorders in executive functions, visual memory, attention and social cognition. Prog. Neuropsychopharmacol. Biol. Psychiatry80(Pt C), 168–176 (2018). - PubMed
-
- Fiorillo, A. et al. Lurasidone in adolescents and adults with schizophrenia: From clinical trials to real-world clinical practice. Expert Opin. Pharmacother.23, 1801–1818 (2022). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

