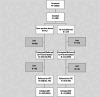
Efforts to link HIV-positive and high-risk blood donors to HIV testing, and treatment services, Mozambique, 2019-2020
- PMID: 40595840
- PMCID: PMC12218381
- DOI: 10.1038/s41598-025-03259-2
Efforts to link HIV-positive and high-risk blood donors to HIV testing, and treatment services, Mozambique, 2019-2020
Abstract
Mozambique's National Blood Transfusion Services (NBTS) is tasked with providing safe and available blood but also conducting systematic screening of at-risk potential donors, notifying seropositive blood donors, and linking them to HIV care and treatment services. Potential blood donors who were deferred from donating following a behavioral risk screening and all blood donors who screened seropositive for HIV were notified and offered linkage to HIV testing, care, and treatment services by community-based organizations. A prospective study among HIV-positive blood donors and deferred donors was conducted from May 2019 to July 2020 at Maputo Central Hospital Blood Bank and the National Reference Blood Center. The associations between testing, initiating care and treatment services among HIV-positive blood donors and prospective deferred donors were estimated using fully Bayesian multivariable logistic models and odds ratios. Among 885 prospective blood donors enrolled, 173 (20%) were deferred due to self-reported high-risk behaviors identified through a screening questionnaire, and 712 (80%) passed the behavioral-risk screening tool, donated, and the blood donation tested positive for HIV. There were more than 2.5 times as many male donors as female donors with a positive HIV test, and among the deferred donors, more than 84% were males. 36% (256/712) of seropositive donors and 35% (61/173) of deferred donors were referred to HIV testing services. 62% (158/256) of seropositive donors and 4.9% (3/61) of deferred donors who were successfully referred were linked to care and treatment services, and 96% (152/158) of these seropositive donors and 100% (3/3) of deferred as high-risk donors initiated antiretroviral therapy (ART). Of the three service organizations used, one outperformed the other two in linking seropositive donors to ART treatment. The NBTS can serve as a critical entry point for identifying HIV-positive persons. Improved implementation of risk behavior screening tools is needed and could contribute to early identification and initiation of ART for potential donors. Innovative strategies and solutions by community-based organizations can be used to improve blood donor notification and linkage to HIV testing and treatment services.
Keywords: Blood donors; HIV; Linkage; Transfusions.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Consent for publication: Not Applicable. Ethical approval: This activity was reviewed by the U.S. Centers for Disease Control and Prevention, deemed not research, and was conducted consistent with applicable federal law and CDC policy (45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. Section 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Section 3501 et seq). The protocol was approved by the Mozambique Ethics Review Board.
Figures
References
-
- World Health Organization (WHO). Blood transfusion safety: Global Collaboration for Blood Safety (2000–2010) 2020. Accessed June 15 (2020). https://www.who.int/bloodsafety/gcbs/en/
-
- WHO. Blood Transfusion Safety. Geneva (2008).
-
- World Health Organization, Wentworth, W. H. O., Mocroft, D., Smart, A. & Wallrauch, I. Global status report on road safety 2015. PLoS One. 53, 833–842 (2015). https://doi.org/ISBN 978 92 4 156506 6.
-
- World Health Organization. Global Database on Blood Safety. Geneva (2011).
-
- Takei, T., Amin, N. A., Schmid, G., Dhingra-Kumar, N. & Rugg, D. Progress in global blood safety for HIV. J Acquir Immune Defic Syndr 2009;52:127–31. 10.1097/QAI.0b013e3181baf0ac (1988). - PubMed
MeSH terms
Grants and funding
- GH12-125505CONT17 (AABB/U.S. President's Emergency Plan for AIDS Relief
- GH12-125505CONT17 (AABB/U.S. President's Emergency Plan for AIDS Relief
- GH12-125505CONT17 (AABB/U.S. President's Emergency Plan for AIDS Relief
- GH12-125505CONT17 (AABB/U.S. President's Emergency Plan for AIDS Relief
- GH12-125505CONT17 (AABB/U.S. President's Emergency Plan for AIDS Relief
- GH12-125505CONT17 (AABB/U.S. President's Emergency Plan for AIDS Relief
- GH12-125505CONT17 (AABB/U.S. President's Emergency Plan for AIDS Relief
- GH12-125505CONT17 (AABB/U.S. President's Emergency Plan for AIDS Relief
- GH12-125505CONT17 (AABB/U.S. President's Emergency Plan for AIDS Relief
- GH12-125505CONT17 (AABB/U.S. President's Emergency Plan for AIDS Relief
- GH12-125505CONT17 (AABB/U.S. President's Emergency Plan for AIDS Relief
- GH12-125505CONT17 (AABB/U.S. President's Emergency Plan for AIDS Relief
- GH12-125505CONT17 (AABB/U.S. President's Emergency Plan for AIDS Relief
LinkOut - more resources
Full Text Sources
Medical