Efficacy and safety of stem cell therapy for acute and subacute ischemic stroke: a systematic review and meta-analysis
- PMID: 40595869
- PMCID: PMC12217828
- DOI: 10.1038/s41598-025-04405-6
Efficacy and safety of stem cell therapy for acute and subacute ischemic stroke: a systematic review and meta-analysis
Abstract
The efficacy of stem cell therapy for ischemic stroke in terms of functional outcomes remains unclear. We conducted a systematic review and meta-analysis of randomized controlled trials (PROSPERO: CRD42024503763) to assess the efficacy and safety of stem cell therapy for acute/subacute ischemic stroke, focusing on long-term outcomes. Studies of patients undergoing stem cell transplantation within 1 month of stroke onset were included. We searched five databases for publications up to January 17, 2024. Summary data were extracted from published reports. The primary outcome was the modified Rankin Scale (mRS) score. Measures of effect were risk ratios (RRs) with 95% confidence intervals (CIs). A random-effects model was used when I2 was > 25%; otherwise, a fixed-effects model was used. Common serious adverse events were epilepsy, gastrointestinal disorders, and cardiac disorders. The risk of bias was assessed using the Cochrane Risk of Bias tool version 2. In total, 13 trials involving 872 (519 men) patients were included. The 1-year incidence of mRS scores 0-1 was higher in the cell-therapy group (45/195) than that in the control group (23/179; RR = 1.74 [95% CI = 1.09-2.77]; p = 0.020; I2 = 0%). The 90-day incidence of mRS scores 0-2 was also higher (RR = 1.31 [95% CI = 1.01-1.70]; p = 0.044; I2 = 0%). No significant differences were observed in serious adverse events or mortality. Stem cell therapy for acute/subacute ischemic stroke within 1 month of onset is safe and significantly improves long-term functional outcomes, although the mechanisms of action need to be elucidated and treatment protocols standardized to establish stem cell therapy as a standard care option for ischemic stroke.
Keywords: Assessment; Ischemic stroke; Outcome; Stem cell transplantation; Systematic review.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: TO received a travel allowance from Healios. KH received consulting fees from Healios. ST, YM, KO, and MF declare no conflicts of interests. Disclosures: TO received a travel allowance from Healios. KH received consulting fees from Healios. ST, YM, KO, and MF declare no conflicts of interests.
Figures
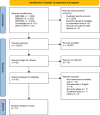



Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Pathogen-reduced platelets for the prevention of bleeding.Cochrane Database Syst Rev. 2013 Mar 28;(3):CD009072. doi: 10.1002/14651858.CD009072.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Jul 30;7:CD009072. doi: 10.1002/14651858.CD009072.pub3. PMID: 23543569 Updated.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD010216. doi: 10.1002/14651858.CD010216.pub7. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 Jan 8;1:CD010216. doi: 10.1002/14651858.CD010216.pub8. PMID: 36384212 Free PMC article. Updated.
References
-
- Feigin, V. L. et al. World stroke organization (WSO): global stroke fact sheet 2022. Int. J. Stroke. 17, 18–29 (2022). - PubMed
-
- Stapf, C. & Mohr, J. P. Ischemic stroke therapy. Annu. Rev. Med.53, 453–475 (2002). - PubMed
-
- Borlongan, C. V., Tajima, Y., Trojanowski, J. Q., Lee, V. M. & Sanberg, P. R. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp. Neurol.149, 310–321 (1998). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

