Cold induced expression of a novel levansucrase gene sacB1 enhances exopolysaccharide production and stress resilience in Leuconostoc mesenteroides
- PMID: 40595955
- PMCID: PMC12215647
- DOI: 10.1038/s41598-025-04141-x
Cold induced expression of a novel levansucrase gene sacB1 enhances exopolysaccharide production and stress resilience in Leuconostoc mesenteroides
Abstract
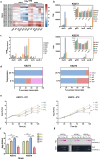
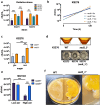
Exopolysaccharides (EPS) play critical roles in microbial survival, stress adaptation, and biofilm formation across diverse environments. In food-associated bacteria such as Leuconostoc mesenteroides, understanding the regulation of EPS production under environmental stress is important for both spoilage control and industrial applications. However, the mechanisms linking cold stress to EPS biosynthesis remain poorly understood. Here, we show that sucrose and low temperature (8 °C) trigger a metabolic shift from dextran-only to combined dextran and levan biosynthesis in four meat-borne Leuc. mesenteroides strains. Two high EPS-producing strains (HEPRs) possess the sacB_1 gene, which encodes a previously uncharacterized levansucrase absent from low EPS-producing strains (LEPRs) that only carry the levS gene. This is the first study to describe the role of sacB_1 in cold-induced EPS production. Notably, sacB_1 was also identified in Leuc. mesenteroides strains isolated from plant-based fermentations such as kimchi and birch sap, but the HEPR strains analyzed here are the only known meat-derived isolates to carry this gene. Genomic analyses revealed highly conserved biosynthetic clusters for dextran, heteropolysaccharide, and levan. Gene expression profiling showed that levS and sacB_1 were upregulated at 8 °C, while dsrD expression was favoured at 25 °C. Cold-induced sucrose metabolism, characterized by high expression of levS, sacB_1, and dsrD, enhanced cell viability under oxidative stress. Furthermore, heterologous expression of sacB_1 in Leuc. mesenteroides and Lactococcus lactis improved resilience under cold and high-aeration conditions, confirming the protective role of levan. These findings advance the understanding of temperature-dependent EPS regulation in LAB and highlight sucrase diversity as a key factor in microbial adaptation to environmental stress.
Keywords: Leuconostoc mesenteroides; Cold stress; Exopolysaccharide; Levansucrase; Sucrose-induced metabolism.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Whitfield, C. & Roberts, I. S. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol.31, 1307–1319 (1999). - PubMed
-
- Flemming, H. C. et al. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol.14, 563–575 (2016). - PubMed
-
- Inuki, S. et al. Chemical synthesis and evaluation of exopolysaccharide fragments produced by Leuconostoc mesenteroides strain NTM048. Chem. Pharm. Bull. (Tokyo). 70, 155–161 (2022). - PubMed
-
- Zannini, E., Waters, D. M., Coffey, A. & Arendt, E. K. Production, properties, and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl. Microbiol. Biotechnol.100, 1121–1135 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

