THSG counteracts microglial glycolytic reprogramming and neuronal necroptosis both in vivo and in vitro under conditions of neuroinflammation
- PMID: 40596006
- PMCID: PMC12216559
- DOI: 10.1038/s41598-025-05994-y
THSG counteracts microglial glycolytic reprogramming and neuronal necroptosis both in vivo and in vitro under conditions of neuroinflammation
Abstract
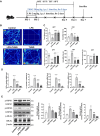
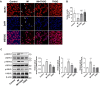
Microglial activation, driven by a metabolic shift towards aerobic glycolysis, is implicated in neuroinflammation and neurological disorders like depression. THSG (2,3,5,4'-Tetrahydroxystilbene-2-O-β-D-glucoside), derived from Polygonum multiflorum, exhibits anti-inflammatory and neuroprotective properties, but its mechanisms, particularly its impact on microglial metabolism, are largely unexplored. Using a LPS-induced mouse model of neuroinflammation, we observed that THSG significantly ameliorated depression-like behaviors. It suppressed pro-inflammatory cytokine production (TNF-α, IL-1β, IL-6, iNOS), inhibited microglial activation, and reduced key necroptosis markers (phosphorylated RIPK1, RIPK3, and MLKL) in the hippocampus. Importantly, THSG effectively suppressed LPS-induced activation of the glycolytic pathway in the hippocampus, as evidenced by increased ATP levels, decreased lactate levels, reduced activity of key glycolytic enzymes, and decreased expression of PKM2 and HIF-1α, critical players in microglial glycolysis. Further in vitro studies with BV2 microglial cells confirmed that THSG significantly suppressed glycolytic enhancement, promoting a metabolic shift towards oxidative phosphorylation, thus inhibiting inflammatory activation of microglia. Co-culture experiments of BV2 cells and SH-SY5Y cells further corroborated the in vivo findings, demonstrating that THSG mitigated inflammation-induced necroptosis in SH-SY5Y neurons by reducing phosphorylation of RIPK1, RIPK3, and MLKL, thus protecting neurons from damage. Our results highlight the potential of THSG as a therapeutic agent for neuroinflammatory disorders by modulating microglial metabolic reprogramming and inhibiting neuronal necroptosis.
Keywords: Depression; Metabolic reprogramming; Microglia; Necroptosis; Neuroinflammation; THSG.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

