Codon-deoptimized single-round infectious virus for therapeutic and vaccine applications
- PMID: 40596017
- PMCID: PMC12215377
- DOI: 10.1038/s41598-025-05643-4
Codon-deoptimized single-round infectious virus for therapeutic and vaccine applications
Abstract
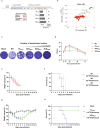
Coxsackievirus B3 (CVB3) is a major cause of myocarditis and acute pancreatitis, particularly in neonates, in whom infections result in severe symptoms and high mortality rates. Despite the urgent need for effective preventive strategies, no vaccines or therapeutic agents have been developed. Live-attenuated vaccines hold promise for combating viral infections; however, their pathogenicity must be carefully regulated without compromising immunogenicity. Here, we investigated codon deoptimization and defective viral genomes (DVGs) as strategies to modulate CVB3 pathogenicity, while preserving its immune-activating capacity. Codon-deoptimized CVB3s with increased CpG dinucleotide content in their 3CD region were engineered, leveraging the innate immunostimulatory properties of CpG. These modified CVB3s exhibited attenuated pathogenicity proportional to the level of codon deoptimization and induced protective immunity against wild-type CVB3 (CVB3WT), making them viable live-attenuated vaccine candidates. Additionally, DVGs derived from codon-deoptimized CVB3 demonstrated superior viral interference and enhanced stimulation of neutralizing antibody production compared to DVGs derived from CVB3WT. These findings highlight that CpG-enriched genomes and DVGs are promising tools for regulating viral pathogenicity, enhancing vaccine safety, and developing therapeutic strategies against viral infections.
Keywords: Codon deoptimization; Coxsackievirus B3; CpG dinucleotide; Defective viral genomes; Live-attenuated vaccines.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: T.N., T.S., P.M., and H.E. are employees of BIKEN, with H.E. holding a managerial position. A.M. declares no competing interests. Appropriate measures were taken to ensure that these affiliations did not influence the study’s design, data collection, analysis, or interpretation. Ethics approval: All experiments were carried out in accordance with relevant guidelines and regulations. This study is reported in accordance with ARRIVE guidelines.
Figures

References
-
- Garmaroudi, F. S. et al. Coxsackievirus B3 replication and pathogenesis. Futur. Microbiol.10, 629–653. 10.2217/fmb.15.5 (2015). - PubMed
-
- Carre, A., Vecchio, F., Flodstrom-Tullberg, M., You, S. & Mallone, R. Coxsackievirus and type 1 diabetes: Diabetogenic mechanisms and implications for prevention. Endocr. Rev.44, 737–751. 10.1210/endrev/bnad007 (2023). - PubMed
-
- Deng, H. et al. An intranasal attenuated coxsackievirus B3 vaccine induces strong systemic and mucosal immunity against CVB3 lethal challenge. J. Med. Virol.96, e29831. 10.1002/jmv.29831 (2024). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

