Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 MPro protein - an in silico and cell-based approach
- PMID: 40596019
- PMCID: PMC12216938
- DOI: 10.1038/s41598-025-05907-z
Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 MPro protein - an in silico and cell-based approach
Abstract
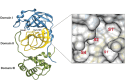
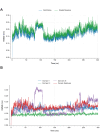
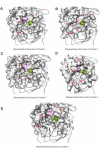
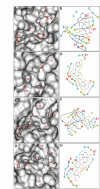
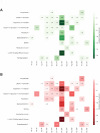
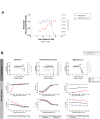
The main protease (MPro) of SARS-CoV-2 plays a crucial role in viral replication and is a prime target for therapeutic interventions. Phytochemicals, known for their antiviral properties, have been previously identified as potential MPro inhibitors in several in silico studies. However, the efficacy of these remains in question owing to the inherent flexibility of the MPro binding site, posing challenges in selecting suitable protein structures for virtual screening. In this study, we conducted an extensive analysis of the MPro binding pocket, utilizing molecular dynamics (MD) simulations, principal component analysis (PCA) and free energy landscape (FEL) to explore its conformational diversity. Based on pocket volume and shape-based clustering, five representative protein conformations were selected for virtual screening. Virtual screening of a library of ~ 48,000 phytochemicals suggested 39 phytochemicals as potential MPro inhibitors. Based on subsequent MM-GBSA binding energy calculations and ADMET property predictions, five compounds were advanced to cell-based viral replication inhibition assays, with three compounds (demethoxycurcumin, shikonin, and withaferin A) exhibiting significant (EC50 < 10 μm) inhibition of SARS-CoV-2 replication. Our study provides an understanding of the binding interactions between these phytochemicals and MPro, contributing significantly to the identification of promising MPro inhibitors. Furthermore, beyond its impact on therapeutic development against SARS-CoV-2, this research highlights a crucial role of proper nutrition in the fight against viral infections.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

