Antigenicity analysis of the recombinant outer membrane NlpI protein of Mannheimia haemolytica
- PMID: 40596169
- PMCID: PMC12218578
- DOI: 10.1038/s41598-025-05125-7
Antigenicity analysis of the recombinant outer membrane NlpI protein of Mannheimia haemolytica
Abstract
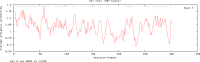
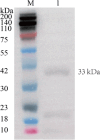
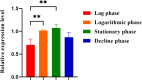
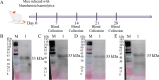
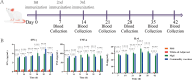
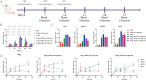
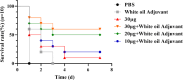
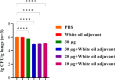
To explore the antigenic characteristics of the NlpI protein. This study screened a potential antigen of Mannheimia haemolytica using NCBI Blast analysis and investigated its immunogenicity using online software. The recombinant NlpI protein (rNlpI) was expressed in prokaryotic cells, and its reactogenicity was detected by western blot. The transcription level of nlpI mRNA in M. haemolytica after various culture durations was determined by RT-qPCR. Mice were challenged with M. haemolytica strain 95 at an LD50 dose, and their serum was collected at various times. The rNlpI protein in M. haemolytica and western blot were used to detect the expression of antibodies specific to rNlpI. BALB/c mice were immunized with various protein doses and white oil adjuvant on days 0, 7, and 14, and the antibody titer was detected by indirect ELISA. Cytokines were detected by double antibody sandwich ELISA. Seven days after the third immunization, M. haemolytica strain 95 was injected intraperitoneally at 3 × 108 CFU/mouse. After 48 h, the surviving mice were dissected and the bacterial load in their lungs was assessed. NlpI is a hydrophilic and soluble protein with a transmembrane domain, 11 antigenic determinants, and 27 B cell epitopes. It was speculated that the protein has strong immunogenicity. The expressed NlpI protein could bind to bovine serum positive for M. haemolytica. The transcription level of the nlpI gene was the highest in the stable phase, followed in decreasing order by the logarithmic, slow, and decline phases. The expression levels in the various phases differed insignificantly. The IgG antibody titers of mice immunized with 10, 20, and 30 µg NlpI + white oil adjuvant and 30 µg NlpI were measured. The antibody level of the 30 µg NlpI + white oil adjuvant group was higher than that in the other groups (P < 0.001). The immune protection rates of the 10 µg rNlpI + white oil adjuvant and 30 µg rNlpI groups were under 30%, while they were 50% and 60%, respectively, in the 20 and 30 µg rNlpI + white oil adjuvant groups. The bacterial load of all immunized groups was 99.99% lower than in the PBS and white oil adjuvant control groups. The protection rates differed significantly among immunized groups (P < 0.001), with the 30 µg rNlpI + white oil adjuvant group showing the lowest bacterial load in the lungs. The rNlpI protein can react with bovine M. haemolytica positive blood with good reactogenicity, and can induce mice to produce high titers of specific IgG antibodies, effectively resisting homologous strain invasion. The results lay a good foundation for developing new M. haemolytica vaccines.
Keywords: Mannheimia haemolytica; Antigenicity; Bioinformatics characteristic; NlpI protein; Prokaryotic expression; Transcription level.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Feedlot Part IV: health and health management on U.S. feedlots with a capacity of 1000 or more head. USDA-APHIS-VS-CEAH-NAHMS, 2013. (2011).
-
- Death loss in U.S. Cattle and Calves Due To Predator and Nonpredator Causes, 2015 (USDA-APHIS-VS-CEAH-NAHMS, 2017).
MeSH terms
Substances
Grants and funding
- 2023NY03-1/Corps eighth division key areas of science and technology research projects
- 2023NY03-1/Corps eighth division key areas of science and technology research projects
- 2023NY03-1/Corps eighth division key areas of science and technology research projects
- 2023NY03-1/Corps eighth division key areas of science and technology research projects
- 2023NY03-1/Corps eighth division key areas of science and technology research projects
- 2023NY03-1/Corps eighth division key areas of science and technology research projects
- 2023NY03-1/Corps eighth division key areas of science and technology research projects
- 2023NY03-1/Corps eighth division key areas of science and technology research projects
- 2023NY03-1/Corps eighth division key areas of science and technology research projects
- 2023NY03-1/Corps eighth division key areas of science and technology research projects
- 2024AB035/Corps key areas of science and technology research
- 2021AB012/Corps key areas of science and technology research
- 2024AB035/Corps key areas of science and technology research
- 2024AB035/Corps key areas of science and technology research
- 2024AB035/Corps key areas of science and technology research
- 2024AB035/Corps key areas of science and technology research
- 2021AB012/Corps key areas of science and technology research
- 2024AB035/Corps key areas of science and technology research
- 2024AB035/Corps key areas of science and technology research
- 2024AB035/Corps key areas of science and technology research
- 2024AB035/Corps key areas of science and technology research
- 2024AB035/Corps key areas of science and technology research
LinkOut - more resources
Full Text Sources
Research Materials

