Antiviral and anti-inflammatory efficacy of nanoencapsulated brazilian green propolis against SARS-CoV-2
- PMID: 40596171
- PMCID: PMC12217127
- DOI: 10.1038/s41598-025-05683-w
Antiviral and anti-inflammatory efficacy of nanoencapsulated brazilian green propolis against SARS-CoV-2
Abstract
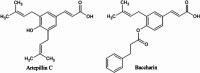
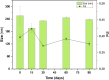
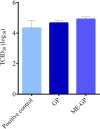
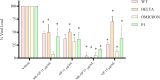
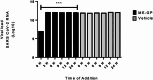
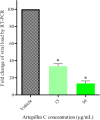
The global COVID-19 pandemic, caused by SARS-CoV-2, continues to pose a significant threat to public health and the economy. SARS-CoV-2 is highly contagious, transmitted primarily through direct contact or inhalation of droplets, and can cause severe respiratory illnesses and other health complications, including post-acute COVID-19 syndrome. This study explored the antiviral potential of Brazilian green propolis, a natural product rich in flavonoids and phenolic compounds encapsulated in a microemulsion, to enhance its stability and antiviral effects. Brazilian green propolis extract was encapsulated in a microemulsion (ME-GP) and characterized using various physicochemical techniques. Furthermore, the antiviral and anti-inflammatory activities of ME-GP was evaluated in vitro and ex-vivo against SARS-CoV-2. For this, cells or tonsils were treated with ME-GP followed by infection with SARS-CoV-2. The microemulsion showed a size of approximately 217 nm, negative zeta potential, high encapsulation efficiency for artepillin C and baccharin (< 99%), and a spherical morphology. The ME-GP formulation was evaluated for antiviral activity against multiple SARS-CoV-2 variants (Wuhan, Gamma, Delta, and Omicron) in Caco-2 cells. The results demonstrated a significant reduction in viral load, particularly for the Wuhan and Delta variants, with up to a 99% reduction in viral load under prophylactic treatment conditions. Time-of-addition assays revealed that ME-GP acts at an early stage in the viral life cycle, likely by interfering with viral entry or immediate post-entry events. Additionally, ME-GP was evaluated in human tonsils, demonstrating an 80% reduction in viral load, suggesting its potential to reduce the transmission and progression of infection. Furthermore, ME-GP exhibited anti-inflammatory activity in human tonsils, significantly decreasing IL-1β, IL-6, TNF-α, and TNF-β levels. Thus, this study highlights the promising prophylactic and therapeutic potential of nanoencapsulated green propolis for combating SARS-CoV-2 and its variants, providing a natural adjunct in COVID-19 therapy.
Keywords: Green propolis; Microemulsion; SARS-CoV-2; Variants.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- WHO Coronavirus (COVID-19) Dashboard. (2023). https://covid19.who.int
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

