ORLA combined with telerehabilitation in patients with subacute Poststroke aphasia: a randomized controlled trial
- PMID: 40596208
- PMCID: PMC12215673
- DOI: 10.1038/s41598-025-06258-5
ORLA combined with telerehabilitation in patients with subacute Poststroke aphasia: a randomized controlled trial
Abstract
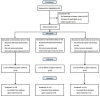
Oral reading for language in aphasia (ORLA) therapy coupled with telerehabilitation (TR) is a promising adjunct to treatment for aphasia. This single-center, randomized, single-blind, 6-month trial aimed to evaluate whether ORLA combined with TR demonstrates comparable efficacy to conventional ORLA offline rehabilitation for improving comprehensive language ability in subacute aphasia patients while assessing longitudinal treatment effects. Enrolled patients were randomized 1:1:1 into Group A (medium-high intensity ORLA TR), Group B (low-intensity ORLA TR), and Group C (medium-high intensity ORLA offline). All groups received a 6-month, 90-h intervention comprising three treatment phases. The primary outcome was a change in Aphasia Quotient (AQ) at baseline and 4 weeks, 3 months, and 6 months post-treatment. The secondary outcomes included quality of life and language subscale scores at the same time points. A total of 42 participants were analyzed, Group A demonstrated a non-significant mean difference of - 0.62 (95% CI - 5.00 to 6.23; P = 0.825) in AQ compared to Group C. A statistically significant difference in the Stroke Aphasia Quality of Life-39 Scale (SAQOL-39) scores between Group A and Group C (mean difference: - 8.19; 95% CI [- 12.39, - 3.99]; P < 0.001). Group A vs. Group C: No statistically significant differences were observed in any of the aforementioned language subskills between Group A and Group C (P > 0.05 for all comparisons). ORLA combined with TR demonstrated comparable efficacy to conventional ORLA offline rehabilitation, serving as a viable alternative for home-based speech rehabilitation in subacute aphasia patients. This TR approach improved both comprehensive language ability and quality of life, with sustained treatment effects over time.First registration date: August 18, 2023 (retrospective registration, Chinese Clinical Trial Registry number: ChiCTR2300074870).
Keywords: Aphasia; Ischemic stroke; Language; Telerehabilitation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval and consent to participate: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee. The procedures and consent form were approved by the Institutional Review Board at Affiliated Sichuan Provincial Rehabilitation Hospital of Chengdu University of TCM. Informed consent: Informed consent was obtained from all individual participants included in the study. Consent for publication: Not applicable.
Figures
References
-
- Laver, K., Walker, M. & Ward, N. Telerehabilitation for stroke is here to stay. But at what cost. Neurorehabil. Neural. Repair36, 331–334 (2022). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical