Engineering M13 bacteriophage to display HER2 mimotopes on pVIII for vaccine development
- PMID: 40596331
- PMCID: PMC12219315
- DOI: 10.1038/s41598-025-08032-z
Engineering M13 bacteriophage to display HER2 mimotopes on pVIII for vaccine development
Abstract
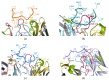
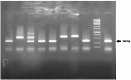
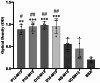
HER2-positive breast cancer is a subtype characterized by overexpression of the human epidermal growth factor receptor 2 (HER2), which is associated with aggressive tumor growth and poor prognosis. Peptide mimics of HER2 epitopes, recognized by tumor growth-inhibitory antibodies like trastuzumab, have the potential to be developed into effective vaccines. Active immunization with these mimotopes could induce tumor-inhibitory antibodies, offering a cost-effective alternative to trastuzumab therapy. This study aimed to design high-affinity mimotopes to trastuzumab using bioinformatics tools and to evaluate the potential of M13 bacteriophages as platforms for displaying these mimotopes. Using the Peptiderive server, peptide sequences involved in binding HER2 to trastuzumab were identified and refined. Docking studies validated their enhanced binding affinity to trastuzumab. The optimized mimotopes were then displayed on the pVIII coat protein of M13 bacteriophages using the PAK8 phagemid vector. ELISA assays confirmed the binding of these recombinant phages to trastuzumab.This study highlights the effectiveness of using bioinformatics tools to enhance mimotope design for therapeutic applications. The M13 bacteriophage displaying optimized mimotopes shows potential as a vaccine candidate for HER2-positive breast cancer, offering a promising approach to overcoming current limitations of trastuzumab therapy.
Keywords: Bacteriophage; HER2; Mimotope; Phage display; Trastuzumab.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Lapatinib and trastuzumab in combination with an aromatase inhibitor for the first-line treatment of metastatic hormone receptor-positive breast cancer which over-expresses human epidermal growth factor 2 (HER2): a systematic review and economic analysis.Health Technol Assess. 2011;15(42):1-93, iii-iv. doi: 10.3310/hta15420. Health Technol Assess. 2011. PMID: 22152751 Free PMC article.
-
Lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer: A systematic review .Int J Clin Pharmacol Ther. 2018 Feb;56(2):72-80. doi: 10.5414/CP203123. Int J Clin Pharmacol Ther. 2018. PMID: 29231164
-
Quantitative [89Zr]Zr-Trastuzumab PET and Diffusion- Weighted MRI for Characterization of Metastatic HER2-Positive Breast Cancer with PET/MRI.J Nucl Med. 2025 Jul 1;66(7):1018-1026. doi: 10.2967/jnumed.124.268931. J Nucl Med. 2025. PMID: 40473459 Free PMC article.
-
Efficacy and safety of lapatinib and trastuzumab for HER2-positive breast cancer: a systematic review and meta-analysis of randomised controlled trials.BMJ Open. 2017 Mar 13;7(3):e013053. doi: 10.1136/bmjopen-2016-013053. BMJ Open. 2017. PMID: 28289045 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

