Biogenic design of silicious architectures on Moso bamboo culm
- PMID: 40596399
- PMCID: PMC12219570
- DOI: 10.1038/s41598-025-06906-w
Biogenic design of silicious architectures on Moso bamboo culm
Abstract
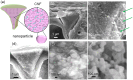
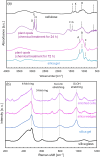
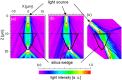
Biosilicas that are produced in vascular plants (plant opal), such as Poaceae, have a variety of shapes and functions and are regarded as an excellent model for the architectural design of artificial amorphous materials. In this work, we studied the micro- and nanostructures and mechanical and optical functions of plant opals on the bamboo culm, which is available as an important natural material. The surface of the culm wall is totally covered with silicified epidermal cells containing silica wedges. The biogenic silicious architectures, such as silicified cell walls and wedges, are composed of nanoscale particles ~ 20-80 nm in diameter with cellulose nanofibrils. Silica wedges, which have a relatively low organic content and relatively high hardness and Young's modulus, are initially formed on cellulose nanofibrils in an organic frame as a scaffold within a few weeks after the emergence of a bamboo shoot. Several months after the formation of the wedges, the epidermal cell walls, which protect the culm surface, are lightly silicified with cellulose nanofibrils. According to a numerical simulation, the silica wedges would have an optical function delivering sunlight to chloroplasts located under the epidermal cells.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Miyajima, R., Oaki, Y., Kogure, T. & Imai, H. Variation in mesoscopic textures of biogenic and biomimetic calcite crystals. Cryst. Growth Des.15, 3755–3761 (2015). - DOI
-
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev.52, 661–699 (2010). - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

