In vitro antiviral activities of thymol and Limonin against influenza a viruses and SARS-CoV-2
- PMID: 40596436
- PMCID: PMC12217544
- DOI: 10.1038/s41598-025-06967-x
In vitro antiviral activities of thymol and Limonin against influenza a viruses and SARS-CoV-2
Abstract
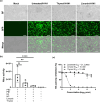
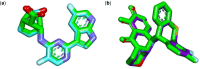
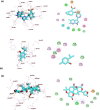
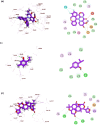
Emerging and re-emerging respiratory viruses represent a continuing threat to human health. The pandemic severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and influenza A viruses (IAVs) are co-circulating, presenting serious threats to public health. Therefore, screening for safe and broad-spectrum antiviral candidates to control such viral infections is prioritized. Herein, this study reports the in vitro antiviral activity of some essential volatile oils (EOs) and volatile oil components including Peppermint oil, Eucalyptus oil, Clove oil, Thymol, Camphor and Limonin against two different IAVs, namely influenza A/H1N1 and A/H5N1 viruses, and SARS-CoV-2 virus. All tested samples were safe in MDCK and Vero E6 cell lines with CC50 values that exceed 1 mg/ml, allowing the screening of their antiviral activities using a wide range of concentrations. The results show the potency of Thymol and Limonin against influenza A/H1N1 virus with IC50 values of 0.022 and 4.25 µg/ml, respectively. The anti-influenza activities of Thymol and Limonin were further validated by testing them against the avian influenza A/H5N1 virus, resulting in anti-influenza activities with IC50 values of 18.5 and 15.6 ng/ml, respectively. The broad-spectrum potential of the highly potent antiviral candidates, Thymol and Limonin, were further tested against the pandemic SARS-CoV-2 and, both exerted anti-coronavirus activities with IC50 values of 0.591 and 4.04 µg/ml, respectively. Further investigations against influenza A/H1N1 virus revealed that Thymol and Limonin could inhibit IAV by hindering viral replication. The Biochemical analyses of the interaction of Limonin and Thymol with FDA-approved anti-influenza drug targets, neuraminidase and viral polymerases, revealed that both compounds can partially inhibit IAV polymerase activity, but have no effect on neuraminidase activity. Likely, molecular docking studies indicated that Thymol and Limonin obstruct active binding sites of IAV polymerases. These findings presented on the antiviral activity of Limonin and Thymol might be used to support the development of supplemental therapy against currently emerging and reemerging respiratory viral infections.
Keywords: Essential oils; IAV; Limonin; Respiratory viruses; SARS-CoV-2; Thymol.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
-
- Boncristiani, H., Criado, M. & Arruda, E. Respiratory viruses. Encyclopedia Microbiology 500. (2009).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

