Follicle architecture and innervation of functionally distinct rat vibrissae
- PMID: 40596464
- PMCID: PMC12216610
- DOI: 10.1038/s42003-025-08336-w
Follicle architecture and innervation of functionally distinct rat vibrissae
Abstract
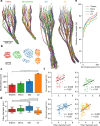
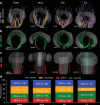
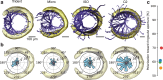
The vibrissa follicle is a complex mechanotransducer with intricate accessory structures such as vibrissa, ring sinus and ringwulst as well as rich innervation by diverse afferent types. Establishing how afferent types and accessory structures operate together to derive specific kinds of sensory information has been challenging, because we often lack precise information on afferent types, accessory structures and vibrissa function. Here we address this challenge by synchrotron X-ray imaging of vibrissa follicles of rat vibrissae with distinct function. Specifically, we characterize accessory structures and trace myelinated axons of the all-purpose-sensing C2-, an object-sensing micro-, the wind-sensing supraorbital- and the ground-sensing trident-vibrissa. We find that while vibrissa length and follicle size differ widely across these vibrissae, the ringwulst and the associated club-like afferents are of near constant diameter and height and appear to form a non-scalable sensory module. The two longer vibrissae (supraorbital and C2 vibrissa) have noticeably more club like afferents, suggesting a special role of the ringwulst in transducing presumably smaller deflection angles encountered by long sensory hairs. The trident vibrissa receives overall few afferents, which are strongly polarized to the posterior vibrissa-shaft, a putative specialization to sensing forward-egomotion. We conclude that high-resolution structural analysis allows relating follicle architecture and function.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Three-dimensional architecture and linearized mapping of vibrissa follicle afferents.Nat Commun. 2025 Jan 8;16(1):499. doi: 10.1038/s41467-024-55468-4. Nat Commun. 2025. PMID: 39779697 Free PMC article.
-
Multiple cortical systems influence a single vibrissa muscle.Proc Natl Acad Sci U S A. 2025 Jun 10;122(23):e2503325122. doi: 10.1073/pnas.2503325122. Epub 2025 Jun 3. Proc Natl Acad Sci U S A. 2025. PMID: 40460131 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Rice, F. L., Mance, A. & Munger, B. L. A comparative light microscopic analysis of the sensory innervation of the mystacial pad. I. Innervation of vibrissal follicle-sinus complexes. J. Comp. Neurol.252, 154–174 (1986). - PubMed
-
- Ebara, S., Kumamoto, K., Matsuura, T., Mazurkiewicz, J. E. & Rice, F. L. Similarities and differences in the innervation of mystacial vibrissal follicle-sinus complexes in the rat and cat: a confocal microscopic study. J. Comp. Neurol.449, 103–119 (2002). - PubMed
-
- Fundin, B. T., Arvidsson, J. & Rice, F. L. Innervation of nonmystacial vibrissae in the adult rat. J. Comp. Neurol.357, 501–512 (1995). - PubMed
MeSH terms
Grants and funding
- EXC-2049 - 390688087/Deutsche Forschungsgemeinschaft (German Research Foundation)
- I-20220980/Deutsches Elektronen-Synchrotron (DESY)
- I-20240117/Deutsches Elektronen-Synchrotron (DESY)
- I-20220980/Deutsches Elektronen-Synchrotron (DESY)
- I-20240117/Deutsches Elektronen-Synchrotron (DESY)
- I-20220980/Deutsches Elektronen-Synchrotron (DESY)
- I-20240117/Deutsches Elektronen-Synchrotron (DESY)
- I-20220980/Deutsches Elektronen-Synchrotron (DESY)
- I-20240117/Deutsches Elektronen-Synchrotron (DESY)
- I-20220980/Deutsches Elektronen-Synchrotron (DESY)
- I-20240117/Deutsches Elektronen-Synchrotron (DESY)
- I-20220980/Deutsches Elektronen-Synchrotron (DESY)
- I-20240117/Deutsches Elektronen-Synchrotron (DESY)
LinkOut - more resources
Full Text Sources
Miscellaneous

