CXCL10-induced regulatory T cells and adenosine signaling promote immunosuppression and progression of epithelial ovarian cancer
- PMID: 40596479
- PMCID: PMC12217468
- DOI: 10.1038/s41598-025-06812-1
CXCL10-induced regulatory T cells and adenosine signaling promote immunosuppression and progression of epithelial ovarian cancer
Erratum in
-
Correction: CXCL10-induced regulatory T cells and adenosine signaling promote immunosuppression and progression of epithelial ovarian cancer.Sci Rep. 2025 Jul 23;15(1):26787. doi: 10.1038/s41598-025-12693-1. Sci Rep. 2025. PMID: 40702101 Free PMC article. No abstract available.
Abstract
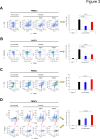
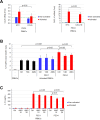
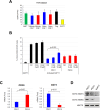
Epithelial ovarian cancer (EOC) is characterized by a highly immunosuppressive tumor microenvironment (TME) that enables EOC progression and limits the efficacy of current immunotherapies. In this study, we demonstrated that isogenic BRCA2-mutated PEO1 and BRCA2-wild type PEO4 EOC cells induced immunosuppressive TMEs through distinct mechanisms. PEO1 cells produced IFNγ-induced PD-L1 and expressed CD39 and CD73 for generating adenosine. Treatment with the adenosine antagonist CGS15943 reversed PEO1 cell-mediated suppression of effector T cell activation. In contrast, PEO4 cells secreted IFNγ-induced CXCL10 and promoted up-regulation of FOXP3+ regulatory T cells (Tregs). Treatment with the CXCL10/CXCR3 antagonist AMG487 attenuated PEO4 cell-induced Tregs and decreased IL10 production. In vivo, administration of a monoclonal antibody against CXCR3 effectively hindered the progression of tumor ascites and prolonged survival in the p53(-/-) ID8 EOC syngeneic mouse model. Additionally, AMG487 treatment synergized with the VEGFA inhibitor bevacizumab, significantly reducing tumor ascites and extending mouse survival. Collectively, our results reveal that EOC leverages CXCL10-induced Tregs or adenosine signaling to dampen T cell-mediated anti-cancer immune responses. These findings suggest that targeting CXCL10/CXCR3 and adenosine signaling could effectively counter immunosuppression of EOC, offering a promising therapeutic strategy for improving patient outcomes.
Keywords: Adenosine; CXCL10; CXCR3; Epithelial ovarian cancer; Immunosuppression; Regulatory T cells.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





References
-
- Siegel, R. L., Miller, K. D., Fuchs, H. E., Jemal, A. & Cancer statistics CA: A Cancer Journal for Clinicians 72, 7–33, (2022). 10.3322/caac.21708 (2022). - PubMed
-
- Kim, R., Emi, M., Tanabe, K. & Arihiro, K. Tumor-Driven evolution of immunosuppressive networks during malignant progression. Cancer Res.66, 5527–5536 (2006). - PubMed
-
- Zhang, Y. & Zheng, J. in Regulation of Cancer Immune Checkpoints: Molecular and Cellular Mechanisms and Therapy (ed Jie Xu) 201–226Springer Singapore, (2020).
-
- Spranger, S. & Gajewski, T. F. Mechanisms of tumor Cell–Intrinsic immune evasion. Annual Rev. Cancer Biology. 2, 213–228. 10.1146/annurev-cancerbio-030617-050606 (2018).
-
- Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint Blockade therapy. Cancer Discov.8, 1069–1086. 10.1158/2159-8290.cd-18-0367 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

