A comparative study of GVHD prophylaxis using low dose ATG versus PTCy for PBSCT based on two independent prospective cohorts
- PMID: 40596499
- PMCID: PMC12216378
- DOI: 10.1038/s41598-025-07263-4
A comparative study of GVHD prophylaxis using low dose ATG versus PTCy for PBSCT based on two independent prospective cohorts
Abstract
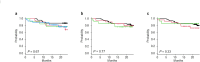
Both anti-thymocyte globulin (ATG) and post-transplant cyclophosphamide (PTCy) have shown prophylactic effects on graft-versus-host disease (GVHD) in multiple phase III studies. We conducted a comparative study of for low dose ATG (thymoglobulin) versus PTCy for GVHD prophylaxis in peripheral blood stem cell transplantation (PBSCT). The ATG (n = 67) and PTCy (n = 40) groups included patients enrolled in multicenter phase II studies (JSCT-ATG15 and JSCT-PTCY19, respectively). The probability of GVHD-free and relapse-free survival at 2 years as the primary endpoint was not significantly different between these two groups (57.9% for ATG vs. 67.8% for PTCy, P = 0.49). Both neutrophil and platelet engraftments were both significantly shorter in the ATG group than in the PTCy group (neutrophils: median 13 days vs. 15 days, P = 0.007; platelets: median 20 days vs. 27 days, P = 0.007). The cumulative incidences of acute and chronic GVHD, relapse, non-relapse mortality, and off-immunosuppressant use were similar between these two groups. The probabilities of overall and progression-free survival were 83.4% and 70.0% in the ATG group and 76.5% and 75.2% in the PTCy group, respectively, with no significant differences. These data indicate that low dose ATG and PTCy are equivalent for GVHD prophylaxis for PBSCT.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: TT reports grants from Sanofi and donations from SHIONOGI, during the conduct of the study. All the remaining authors declare no conflict of interest.
Figures



Similar articles
-
Outcomes of Antithymocyte Globulin-Post-Transplantation Cyclophosphamide-Cyclosporine-Based versus Antithymocyte Globulin-Based Prophylaxis for 10/10 HLA-Matched Unrelated Donor Allogeneic Hematopoietic Cell Transplantation.Transplant Cell Ther. 2024 May;30(5):536.e1-536.e13. doi: 10.1016/j.jtct.2024.01.075. Epub 2024 Jan 27. Transplant Cell Ther. 2024. PMID: 38281592
-
Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD009159. doi: 10.1002/14651858.CD009159.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2023 Jun 21;6:CD009159. doi: 10.1002/14651858.CD009159.pub3. PMID: 22972135 Updated.
-
Combination of Anti-thymocyte Globulin with Post-transplant Cyclophosphamide for GVHD Prophylaxis in Patients Undergoing Haploidentical Hematopoietic Stem Cell Transplantation: Systematic Review and Meta-analysis.Transplant Cell Ther. 2025 Jan;31(1):32.e1-32.e15. doi: 10.1016/j.jtct.2024.07.017. Epub 2024 Jul 29. Transplant Cell Ther. 2025. PMID: 39084262
-
Sirolimus Is an Acceptable Alternative to Tacrolimus for Graft-versus-Host Disease Prophylaxis after Haploidentical Peripheral Blood Stem Cell Transplantation with Post-Transplantation Cyclophosphamide.Transplant Cell Ther. 2024 Feb;30(2):229.e1-229.e11. doi: 10.1016/j.jtct.2023.11.010. Epub 2023 Nov 11. Transplant Cell Ther. 2024. PMID: 37952648 Clinical Trial.
-
Post-transplant cyclophosphamide versus anti-thymocyte globulin in haploidentical stem cell transplantation: a systematic review and meta-analysis.Ann Hematol. 2025 Mar;104(3):1317-1328. doi: 10.1007/s00277-025-06199-z. Epub 2025 Jan 23. Ann Hematol. 2025. PMID: 39847115 Free PMC article.
References
-
- Socié, G. et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late effects working committee of the international bone marrow transplant registry. N Engl. J. Med.341, 14–21 (1999). - PubMed
-
- Lanzkron, S. M., Collector, M. I. & Sharkis, S. J. Homing of long-term and short-term engrafting cells in vivo. Ann. N Y Acad. Sci.872, 48–54 (1999). discussion 54–56. - PubMed
-
- Bacigalupo, A. et al. Antithymocyte Globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood98, 2942–2947 (2001). - PubMed
-
- Finke, J. et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell Globulin in Haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol.10, 855–864 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

