A comparative study of GVHD prophylaxis using low dose ATG versus PTCy for PBSCT based on two independent prospective cohorts
- PMID: 40596499
- PMCID: PMC12216378
- DOI: 10.1038/s41598-025-07263-4
A comparative study of GVHD prophylaxis using low dose ATG versus PTCy for PBSCT based on two independent prospective cohorts
Abstract
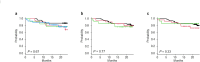
Both anti-thymocyte globulin (ATG) and post-transplant cyclophosphamide (PTCy) have shown prophylactic effects on graft-versus-host disease (GVHD) in multiple phase III studies. We conducted a comparative study of for low dose ATG (thymoglobulin) versus PTCy for GVHD prophylaxis in peripheral blood stem cell transplantation (PBSCT). The ATG (n = 67) and PTCy (n = 40) groups included patients enrolled in multicenter phase II studies (JSCT-ATG15 and JSCT-PTCY19, respectively). The probability of GVHD-free and relapse-free survival at 2 years as the primary endpoint was not significantly different between these two groups (57.9% for ATG vs. 67.8% for PTCy, P = 0.49). Both neutrophil and platelet engraftments were both significantly shorter in the ATG group than in the PTCy group (neutrophils: median 13 days vs. 15 days, P = 0.007; platelets: median 20 days vs. 27 days, P = 0.007). The cumulative incidences of acute and chronic GVHD, relapse, non-relapse mortality, and off-immunosuppressant use were similar between these two groups. The probabilities of overall and progression-free survival were 83.4% and 70.0% in the ATG group and 76.5% and 75.2% in the PTCy group, respectively, with no significant differences. These data indicate that low dose ATG and PTCy are equivalent for GVHD prophylaxis for PBSCT.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: TT reports grants from Sanofi and donations from SHIONOGI, during the conduct of the study. All the remaining authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

