Development and validation of machine learning models for predicting blastocyst yield in IVF cycles
- PMID: 40596532
- PMCID: PMC12217550
- DOI: 10.1038/s41598-025-06998-4
Development and validation of machine learning models for predicting blastocyst yield in IVF cycles
Abstract
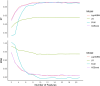
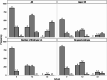
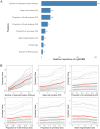
Predicting blastocyst formation poses significant challenges in reproductive medicine and critically influences clinical decision-making regarding extended embryo culture. While previous research has primarily focused on determining whether an IVF cycle can produce at least one blastocyst, less attention has been given to quantifying blastocyst yields. This study aims to develop and validate such a quantitative predictive tool for IVF cycles. We employed three machine learning models-SVM, LightGBM, and XGBoost-which demonstrated comparable performance and outperformed traditional linear regression models (R2: 0.673-0.676 vs. 0.587, Mean absolute error: 0.793-0.809 vs. 0.943). Ultimately, LightGBM emerged as the optimal model, due to utilizing fewer features (8 vs. 10-11 in SVM/XGBoost) and offering superior interpretability. We then stratified predictions and actual yields into three categories (0, 1-2, and ≥ 3 blastocysts) to evaluate the model's discriminative performance. In this multi-classification task, LightGBM demonstrated robust accuracy (0.675-0.71) with fair-to-moderate agreement (kappa coefficients: 0.365-0.5) across both the overall cohort and poor-prognosis subgroups. Feature importance analysis identified three critical predictors: the number of extended culture embryos, the mean cell number on Day 3, and the proportion of 8-cell embryos. By leveraging the potential of machine learning, this research provides clinicians with valuable insights for making individualized decisions regarding extended embryo culture.
Keywords: Blastocyst yield; Clinical decision support; Extended embryo culture; In vitro fertilization; Machine learning.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval for the study was obtained from the Institutional Review Board of Nanfang Hospital, as authorized by the Ethical Committee (approval number: NFEC-2024-326). The procedures followed were in accordance with the ethical standards of the Declaration of Helsinki of the World Medical Association. The Ethical Committee of Nanfang Hospita waived the need for obtaining informed consent from the participants. Competing interests: The authors declare no competing interests.
Figures
References
-
- Glujovsky, D. et al. Cleavage-stage versus blastocyst-stage embryo transfer in assisted reproductive technology. Cochrane Database Syst. Rev.5, Cd002118 (2022). - PubMed
-
- ASRM. Blastocyst culture and transfer in clinically assisted reproduction: a committee opinion. Fertil. Steril.110, 1246–1252 (2018). - PubMed
-
- Smeltzer, S., Acharya, K., Truong, T., Pieper, C. & Muasher, S. Clinical pregnancy and live birth increase significantly with every additional blastocyst up to five and decline after that: an analysis of 16,666 first fresh single-blastocyst transfers from the society for assisted reproductive technology registry. Fertil. Steril.112, 866–873e1 (2019). - PubMed
-
- Xiong, F. et al. Association between the number of top-quality blastocysts and live births after single blastocyst transfer in the first fresh or vitrified-warmed IVF/ICSI cycle. Reprod. Biomed. Online. 40, 530–537 (2020). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

