SARS-CoV-2 bioaerosol transmission in experimentally infected American mink
- PMID: 40596540
- PMCID: PMC12217755
- DOI: 10.1038/s41598-025-08111-1
SARS-CoV-2 bioaerosol transmission in experimentally infected American mink
Abstract
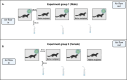
The SARS-CoV-2 BA.1 (Omicron) variant, which emerged in late 2021, is more transmissible than earlier variants but causes milder symptoms in humans. Mink farms, where animals are housed in close quarters, present a high risk for virus transmission and mutation, necessitating strict control measures due to documented cases of mink-to-human and human-to-mink transmission. Hence, we aimed to detect infectious airborne SARS-CoV-2 using BioSampler-air collectors and to investigate aerosol transmission between groups of American mink (Neovison vison). Two groups (male and female) were infected with the BA.1 variant, and samples were collected from aerosols, saliva, feces, and surfaces. The results indicated that infectious viruses were predominantly detected in aerosol samples over a three-day period in both groups. Surface, saliva, and fecal samples also showed potential for virus transmission. Notably, infectious viruses were cultivated from aerosol samples, confirming aerosol transmission among American mink. This study highlights the importance of immediate sample culturing to improve infectious virus detection and emphasizes the need for enhanced preventive measures on mink farms to mitigate the spread of viruses.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: Experimental procedures were approved by the Animal Experimental Board of Finland (ESAVI/33259).
Figures
Similar articles
-
Investigations into SARS-CoV-2 and other coronaviruses on mink farms in France late in the first year of the COVID-19 pandemic.PLoS One. 2023 Aug 25;18(8):e0290444. doi: 10.1371/journal.pone.0290444. eCollection 2023. PLoS One. 2023. PMID: 37624818 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
-
Workplace interventions to reduce the risk of SARS-CoV-2 infection outside of healthcare settings.Cochrane Database Syst Rev. 2022 May 6;5(5):CD015112. doi: 10.1002/14651858.CD015112.pub2. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 Apr 10;4:CD015112. doi: 10.1002/14651858.CD015112.pub3. PMID: 35514111 Free PMC article. Updated.
References
-
- Menni, C. et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of Omicron and delta variant dominance: a prospective observational study from the ZOE COVID study. Lancet399 (10335), 1618–1624. 10.1016/S0140-6736(22)00327-0 (2022). - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 4917/31/2021/Business Finland
- 336490/Research Council of Finland
- 336490/Research Council of Finland
- 336490/Research Council of Finland
- 336490/Research Council of Finland
- 336490/Research Council of Finland
- 336490/Research Council of Finland
- 336490/Research Council of Finland
- 336490/Research Council of Finland
- 336490/Research Council of Finland
- 336490/Research Council of Finland
- 336490/Research Council of Finland
- 336490/Research Council of Finland
- 336490/Research Council of Finland
- 874735/VEO-European Union's Horizon 2020
- 874735/VEO-European Union's Horizon 2020
- 874735/VEO-European Union's Horizon 2020
- 874735/VEO-European Union's Horizon 2020
- 874735/VEO-European Union's Horizon 2020
- 874735/VEO-European Union's Horizon 2020
- 874735/VEO-European Union's Horizon 2020
- 874735/VEO-European Union's Horizon 2020
- 874735/VEO-European Union's Horizon 2020
- 874735/VEO-European Union's Horizon 2020
- 874735/VEO-European Union's Horizon 2020
- 874735/VEO-European Union's Horizon 2020
- 874735/VEO-European Union's Horizon 2020
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

