S100A8 regulated by estrogen improves injured endometrial epithelium reconstruction by promoting tight junction formation and stromal cell transformation
- PMID: 40596550
- PMCID: PMC12219834
- DOI: 10.1038/s41598-025-08530-0
S100A8 regulated by estrogen improves injured endometrial epithelium reconstruction by promoting tight junction formation and stromal cell transformation
Abstract
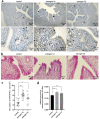
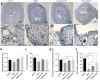
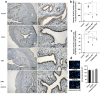
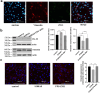
Estrogen is used for endometrial repair; however, it has limited effectiveness. Cytokine S100A8 expression is affected by estrogen and is involved in regulating the damage repair. We aimed to confirm the effects of S100A8 and explore its mechanisms during the reconstruction of the injured endometrium. We investigated the effects of estrogen on S100A8 expression in healthy endometrium. A rat model of endometrial injury and cultured primary endometrial cells were used to determine the pleiotropic effects of S100A8 on endometrial epithelial repair. Estrogen regulated the recruitment of S100A8-positive immune cells (S100A8-PICs) and the release of S100A8 in the healthy endometrium, but estrogen plays a limited role in regulating seriously injured endometrium via self-S100A8. S100A8 administration to the uterine cavity significantly improved the morphology of injured epithelium; influenced the reverse migration of S100A8-PICs; and promoted epithelial localization of proliferating cells, cell junction formation, and transformation of stromal cells to epithelial cells. S100A8 in the uterine cavity has pleiotropic effects that improve endometrial epithelial reconstruction and can compensate for the deficiency in estrogen repair regulation.
Keywords: Cell junction; Endometrial injury; Epithelium reconstruction; Estrogen; S100A8; Transformation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Institutional review board statement: The protocol was approved by the Medical Ethical Committee of Inner Mongolia Medical University (approval number: YKD202302082).
Figures

Similar articles
-
Endometrial changes during ulipristal acetate use: A systematic review.Eur J Obstet Gynecol Reprod Biol. 2017 Jul;214:56-64. doi: 10.1016/j.ejogrb.2017.04.042. Epub 2017 Apr 28. Eur J Obstet Gynecol Reprod Biol. 2017. PMID: 28482329
-
Pre-operative endometrial thinning agents before endometrial destruction for heavy menstrual bleeding.Cochrane Database Syst Rev. 2002;(3):CD001124. doi: 10.1002/14651858.CD001124. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2014 Jul 29;(7):CD001124. doi: 10.1002/14651858.CD001124.pub2. PMID: 12137619 Updated.
-
MMP3 mediates E2-induced bovine endometrial cell proliferation by releasing HB-EGF.J Reprod Dev. 2025 Aug 1;71(4):217-225. doi: 10.1262/jrd.2025-021. Epub 2025 Jul 5. J Reprod Dev. 2025. PMID: 40619252 Free PMC article.
-
Estradiol inhibits endometrial injury by promoting the stability of the KLF15 protein and the recovery of mitochondrial function.Exp Cell Res. 2025 Jul 15;450(2):114651. doi: 10.1016/j.yexcr.2025.114651. Epub 2025 Jun 11. Exp Cell Res. 2025. PMID: 40513792
-
Endometrial injury for pregnancy following sexual intercourse or intrauterine insemination.Cochrane Database Syst Rev. 2016 Jun 14;(6):CD011424. doi: 10.1002/14651858.CD011424.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2021 Mar 18;3:CD011424. doi: 10.1002/14651858.CD011424.pub3. PMID: 27296541 Updated.
References
-
- Liu, T., He, B. & Xu, X. Repairing and regenerating injured endometrium methods. Reprod. Sci.30, 1724–1736 (2023). - PubMed
-
- Healy, M. W. et al. Intrauterine adhesion prevention after hysteroscopy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol.215, 267-275.e7 (2016). - PubMed
-
- Johary, J., Xue, M., Zhu, X., Xu, D. & Velu, P. P. Efficacy of estrogen therapy in patients with intrauterine adhesions: systematic review. J. Minim. Invasive Gynecol.21, 44–54 (2014). - PubMed
MeSH terms
Substances
Grants and funding
- YKD2023MS039/The Scientific Research Fund of Inner Mongolia Medical University
- NJZZ23003/Scientific Research Program in Universities of Inner Mongolia Autonomous Region
- YKD2022ZD016/The Key Project of Inner Mongolia Medical University
- ZY20241102/Zhiyuan Telent Project of Inner Mongolia Medical University
LinkOut - more resources
Full Text Sources

