Therapeutic evaluation of Martynia annua derived carbon dots in epileptic Drosophila model
- PMID: 40596571
- PMCID: PMC12216562
- DOI: 10.1038/s41598-025-07780-2
Therapeutic evaluation of Martynia annua derived carbon dots in epileptic Drosophila model
Abstract
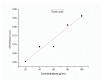
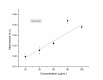
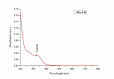
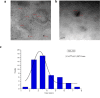
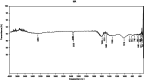
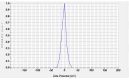
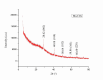
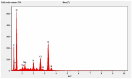
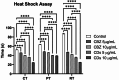
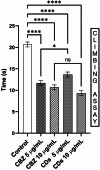
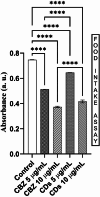
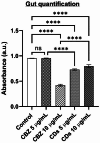
This study investigates the synthesis and characterization of Carbon dots (MA-CDs) derived from the aqueous extract of Martynia annua and examining their potential effects in an epilepsy model Drosophila melanogaster. Phytochemical analysis confirmed the presence of saponin, terpeniods, and flavanoids in the leaf extract, which facilitated the green synthesis of MA-CDs. Physicochemical characterization revealed an absorbance peak at 326 nm, the mean size of the particle was 3.17 ± 0.16 nm, and moderate stability (-1.6 mV). To assess the therapeutic potential of MA-CDs alongside the antiepileptic drug Carbamazepine (CBZ), we conducted behavioral and cognitive assays in para bang senseless (parabss1) mutants of Drosophila, a model organism for epilepsy. Seizures induced by vortex and heat shock were significantly mitigated in a dose-dependent manner in flies treated with both MA-CDs and CBZ. However, higher doses of CBZ and MA-CDs increased the climbing ability of the flies. In cognitive assays, CBZ at higher doses improved memory and learning in mutant flies, while MA-CDs also showed significant impact. MA-CDs were consumed at a higher rate than CBZ when incorporated into food. The green synthesized MA-CDs at its higher concentration has garnered its positive effect on the mutants along with the CBZ antiepileptic drug which also has shown its positive effects when different concentration of them were treated to the mutants.
Keywords: Martynia annua; Carbamazepine; Carbon dots; Epilepsy; Para bang senseless.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval and consent to participate: For experiments involving human participants (including the use of tissue samples), informed consent has been obtained. Written consent was obtained from all individual participants included in the study. Institutional ethical approval was granted by Karnataka Institute for DNA Research (KIDNAR), Dharwad (IEC Ref No. KCTRI/EC-01-A/2024). All procedures performed in studies involving human participants were in accordance with the Helsinki declaration as revised in 2013 and its later amendments.
Figures


Similar articles
-
Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data.Cochrane Database Syst Rev. 2017 Jun 29;6(6):CD011412. doi: 10.1002/14651858.CD011412.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2017 Dec 15;12:CD011412. doi: 10.1002/14651858.CD011412.pub3. PMID: 28661008 Free PMC article. Updated.
-
Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data.Cochrane Database Syst Rev. 2017 Dec 15;12(12):CD011412. doi: 10.1002/14651858.CD011412.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Apr 1;4:CD011412. doi: 10.1002/14651858.CD011412.pub4. PMID: 29243813 Free PMC article. Updated.
-
Levetiracetam add-on for drug-resistant focal epilepsy: an updated Cochrane Review.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD001901. doi: 10.1002/14651858.CD001901.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972056 Free PMC article.
-
Immediate-release versus controlled-release carbamazepine in the treatment of epilepsy.Cochrane Database Syst Rev. 2010 Jan 20;(1):CD007124. doi: 10.1002/14651858.CD007124.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2014 Feb 03;(2):CD007124. doi: 10.1002/14651858.CD007124.pub3. PMID: 20091617 Updated.
-
Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2017 Feb 27;2(2):CD001911. doi: 10.1002/14651858.CD001911.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Jul 18;7:CD001911. doi: 10.1002/14651858.CD001911.pub4. PMID: 28240353 Free PMC article. Updated.
References
-
- Fisher, R. S. et al. Epileptic seizures and epilepsy: Definitions proposed by the international league against epilepsy (ILAE) and the international bureau for epilepsy (IBE). Epilepsia46, 470–472 (2005). - PubMed
-
- Reynolds, E. H. The ILAE/IBE/WHO global campaign against epilepsy: Bringing epilepsy “out of the shadows”. Epilepsy Behav.1, S3–S8 (2000). - PubMed
-
- Beghi, E. & Giussani, G. Aging and the epidemiology of epilepsy. Neuroepidemiology51, 216–223 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

