Design of a plasmonic optical biosensor based on a metal-insulator-metal ring resonator for the detection of various bacterial pathogens
- PMID: 40596579
- PMCID: PMC12215868
- DOI: 10.1038/s41598-025-07331-9
Design of a plasmonic optical biosensor based on a metal-insulator-metal ring resonator for the detection of various bacterial pathogens
Abstract
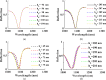
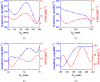
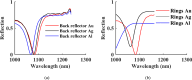
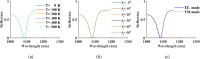
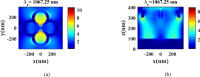
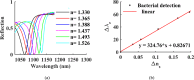
Rapid and sensitive detection of pathogenic bacteria is essential for healthcare, food safety, and environmental monitoring. However, conventional detection techniques often fall short in terms of the speed and sensitivity required for real-time applications. In this study, we propose a label-free plasmonic optical biosensor based on a metal-insulator-metal (MIM) dual-ring resonator structure for the efficient detection of bacterial species. The sensor geometry was optimized using Particle Swarm Optimization (PSO) and evaluated through three-dimensional finite-difference time-domain (3D-FDTD) simulations to enhance both sensitivity and figure of merit (FOM). Gold nanorings integrated with a gold back reflector were employed due to their superior plasmonic resonance characteristics. The optimized design achieved a sensitivity of 324.76 nm·RIU-1, a FOM of 10.187 RIU-1, and a detection limit (LoD) of 0.075 RIU. The biosensor maintained high performance under varying operational conditions, including temperature (0-500 K), incident angle (0°-50°), and polarization states. Strong field confinement in the dielectric gap significantly enhanced the interaction between light and the analyte. The device demonstrated the ability to detect and differentiate between Vibrio cholerae (n = 1.365), Escherichia coli (n = 1.388), and Pseudomonas species (n = 1.437-1.526), highlighting its potential for quantitative bacterial identification. By addressing key limitations in sensitivity and specificity in complex biological environments, this MIM-based sensor offers a robust platform for rapid, high-throughput bacterial detection in clinical diagnostics and beyond.
Keywords: Bacteria; MIM resonator rings; Optical biosensor; Sensitivity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Design and numerical evaluation of a high sensitivity plasmonic biosensor based on MISM nanoring for versatile virus detection.Sci Rep. 2025 Jul 1;15(1):21484. doi: 10.1038/s41598-025-07501-9. Sci Rep. 2025. PMID: 40594778 Free PMC article.
-
Design and optimization of a compact dual band metal insulator metal filter for high sensitivity refractive index sensing using particle swarm optimization.Sci Rep. 2025 Jul 1;15(1):22436. doi: 10.1038/s41598-025-05569-x. Sci Rep. 2025. PMID: 40594519 Free PMC article.
-
Supercell-enhanced multimodal plasmonic sensor for high-fidelity antigen detection via refractive index modulation.Sci Rep. 2025 Jul 1;15(1):22322. doi: 10.1038/s41598-025-08081-4. Sci Rep. 2025. PMID: 40593314 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Bravo-Frank, N. et al. Realtime bacteria detection and analysis in sterile liquid products using deep learning holographic imaging. Npj Biosensing. 1(1), 8 (2024).
-
- Shahbaz, M., Butt, M. A. & Piramidowicz, R. A concise review of the progress in photonic sensing devices. In Photonics, MDPI, 698. Accessed 03 Apr 2025 https://www.mdpi.com/2304-6732/10/6/698 (2023).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

