Circulating MiRNAs as diagnostic biomarkers of lupus nephritis in patients with systemic lupus erythematosus: a systematic review and meta-analysis
- PMID: 40596596
- PMCID: PMC12215461
- DOI: 10.1038/s41598-025-07860-3
Circulating MiRNAs as diagnostic biomarkers of lupus nephritis in patients with systemic lupus erythematosus: a systematic review and meta-analysis
Abstract
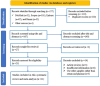
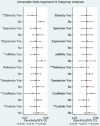
Managing lupus nephritis (LN) has been challenging despite therapeutic advancements, as 30% of patients face end-stage kidney disease. Current laboratory tests for diagnosing LN activity are insufficient. Recognizing the potential for severe complications in patients with LN, there is crucial need to identify innovative biomarkers for early diagnosis of this disease. Hence, the objective of this study was to evaluate diagnostic efficacy of miRNAs for LN. The protocol has been registered on PROSPERO (CRD42023460747). This systematic review and meta-analysis was performed following the PRISMA guidelines. A comprehensive search for relevant literature was carried out across PubMed, Scopus, Embase, and Hinari. The methodological quality of the articles was assessed using the QUADAS-2 tool. Stata 14.0 software was used to calculate pooled sensitivity, specificity, and other diagnostic parameters related to miRNAs for the diagnosis of LN utilizing a random effects model. The assessment of heterogeneity among studies involved the application of the Cochran-Q test and I2 statistic tests. Subsequently, subgroup analyses and meta-regression analysis were conducted to investigate the primary sources of heterogeneity. To evaluate publication bias, Deeks' funnel plot was used. Additionally, Fagan's nomogram and likelihood ratio scattergram were used to assess the clinical utility of miRNAs for LN. Additionally, a sensitivity analysis was conducted to evaluate the robustness and reliability of the results. In this meta-analysis, 23 studies from 11 publications were included, encompassing a total of 526 LN patients and 490 controls. The selected studies pertained low risk of bias. The overall pooled values for sensitivity, specificity, PLR, NLR, DOR, and AUC were 0.85 (95% CI 0.78-0.89), 0.83 (95% CI 0.75-0.89), 4.93 (95% CI 3.47-7.07), 0.19 (95% CI 0.13-0.26), 26.35 (95% CI 16.39-42.36), and 0.91 (95% CI 0.88-0.93), respectively. Furthermore, miR-181a, miR-223 and miR-146a demonstrated superior diagnostic efficacy with promising clinical utility in excluding and confirming LN. Additionally, findings from the subgroup analysis indicated that miRNA panels, upregulated miRNAs, and miRNAs used in the diagnosis of African patients exhibited superior diagnostic performance. Circulating microRNAs (miRNAs) show promise as noninvasive biomarkers for the early diagnosis of LN. Furthermore, miRNA panels and upregulated miRNAs exhibited improved diagnostic efficacy for LN diagnosis. However, substantiating these findings will necessitate a substantial number of prospective studies and multicenter studies in the near future.
Keywords: Diagnostic biomarkers; LN; Lupus nephritis; Meta-analysis; MiRNAs; Noncoding RNAs; Systemic lupus erythematous.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

