Genetic skeletal disorders: phenotypic-genotypic characteristics and RhGH therapy responses of a pediatric cohort
- PMID: 40596606
- PMCID: PMC12216826
- DOI: 10.1038/s41598-025-07570-w
Genetic skeletal disorders: phenotypic-genotypic characteristics and RhGH therapy responses of a pediatric cohort
Abstract
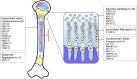
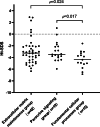
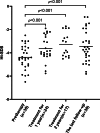
This study aimed to explore the genotype-phenotype correlations in individuals with Genetic Skeletal Disorders (GSD), evaluate the efficacy of recombinant human Growth Hormone (rhGH) therapy. The retrospective analysis of the medical records of 80 pediatric patients with GSD diagnosed via whole-exome sequencing was conducted. The therapeutic effects of rhGH treatment were analyzed in 30 of these patients who received rhGH therapy. The study included 80 GSD patients, diagnosed at a median age of 4.88 years, with a median height standard deviation score (Ht-SDS) of - 3.58. The most common clinical manifestations included skeletal deformities (87.5%), short stature (81.3%), and distinctive facial features (including triangular face, abnormality of the philtrum, abnormality of the forehead, etc.) (65.0%). A total of 33 pathogenic genes associated with 20 groups of GSD were identified. The most common groups are Type II collagenopathies (related to the COL2A1 gene) (12/80, 15.0%) and the FGFR3-related chondrodysplasia group (12/80, 15.0%). Those with pathogenic genes linked to Fundamental Cellular Processes had more severe short stature and prenatal phenotypes. Thirty patients received rhGH treatment for a median of 2.25 years (0.33-8.92), showing Ht-SDS increases of 0.66 ± 0.42 and 0.84 ± 0.52, after one and two years, respectively (p < 0.001). Eight untreated patients had an average Ht-SDS decrease of - 0.46 ± 0.55. In this cohort, pediatric GSD patients predominantly presented with short stature, skeletal deformities, and distinctive facial features (including triangular face, abnormality of the philtrum, abnormality of the forehead, etc.), indicating a genotype-phenotype correlation. Compared to untreated GSD patients, those receiving rhGH treatment demonstrated varying degrees of height improvement, however, the long-term efficacy of this treatment warrants further investigation.
Keywords: Bone diseases; Developmental; Genotype; Growth disorders; Recombinant human growth hormone.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: The present research study is in compliance with Ethical standards and approved from the Second Affiliated Hospital of Guangxi Medical University Ethical Review Committee (2025-KY(0009)).
Figures
Similar articles
-
Recombinant human growth hormone for the treatment of growth disorders in children: a systematic review and economic evaluation.Health Technol Assess. 2010 Sep;14(42):1-209, iii-iv. doi: 10.3310/hta14420. Health Technol Assess. 2010. PMID: 20849734
-
Growth hormone for children with chronic kidney disease.Cochrane Database Syst Rev. 2012 Feb 15;2012(2):CD003264. doi: 10.1002/14651858.CD003264.pub3. Cochrane Database Syst Rev. 2012. PMID: 22336787 Free PMC article.
-
Real-Life Growth Hormone Treatment Patterns in Children from China: A Report from Two Databases.Adv Ther. 2025 Jul;42(7):3562-3575. doi: 10.1007/s12325-025-03204-9. Epub 2025 May 29. Adv Ther. 2025. PMID: 40439954 Free PMC article.
-
Impact of the exon 3-deleted growth hormone (GH) receptor polymorphism on baseline height and the growth response to recombinant human GH therapy in GH-deficient (GHD) and non-GHD children with short stature: a systematic review and meta-analysis.J Clin Endocrinol Metab. 2009 Oct;94(10):3721-30. doi: 10.1210/jc.2009-0425. Epub 2009 Jul 7. J Clin Endocrinol Metab. 2009. PMID: 19584188
-
Recombinant growth hormone therapy for cystic fibrosis in children and young adults.Cochrane Database Syst Rev. 2021 Aug 23;8(8):CD008901. doi: 10.1002/14651858.CD008901.pub5. Cochrane Database Syst Rev. 2021. PMID: 34424546 Free PMC article.
References
-
- Kim, H. Y., Lee, Y. A., Shin, C. H., Cho, T. J. & Ko, J. M. Clinical manifestations and outcomes of 20 Korean hypochondroplasia patients with the FGFR3 n540k variant. Exp. Clin. Endocr. Diab131, 123–131 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

