Locus coeruleus integrity correlates with plasma soluble Axl levels in Alzheimer's disease patients
- PMID: 40596706
- PMCID: PMC12213453
- DOI: 10.1002/alz.70434
Locus coeruleus integrity correlates with plasma soluble Axl levels in Alzheimer's disease patients
Abstract
Introduction: Locus coeruleus (LC) is one of the earliest structures altered in Alzheimer's disease (AD). Inflammation is also now considered critical in AD pathology, early stage included. However, no association between LC degeneration and the peripheral inflammation has been reported yet.
Methods: A cohort of 102 patients was studied for which both magnetic resonance imaging (MRI) scans and blood samples were available. LC integrity was assessed by MRI, and plasma soluble TAMs (Tyro3, Axl, and MerTK) receptor levels were measured by enzyme-linked immunosorbent assay (ELISA).
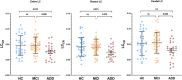
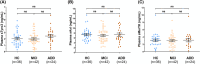
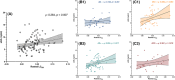
Results: We found that plasma levels of the soluble TAMs receptor Axl were correlated with LC rostral degeneration in the whole cohort (p = 0.007), as well as in the AD+ group (p = 0.017), but not in the AD- group.
Discussion: These results uncover a new relationship between peripheric markers of inflammation and central early AD neurodegeneration.
Highlights: In Alzheimer's disease, no link between locus coeruleus degeneration and microglial activation was reported. Plasma Axl, Tyro3, and MerTK levels and locus coeruleus integrity were assessed in Alzheimer's disease patients. Locus coeruleus integrity positively correlates with plasma AXL, linked to microglia activation. Axl-noradrenergic signaling interplay deserves further larger longitudinal studies.
Keywords: Alzheimer's disease; blood‐based biomarkers; locus coeruleus; neuroinflammation; noradrenaline.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors report no competing interests.
Figures
Similar articles
-
Locus coeruleus integrity and neuropsychiatric symptoms in a cohort of early- and late-onset Alzheimer's disease.Alzheimers Dement. 2024 Sep;20(9):6351-6364. doi: 10.1002/alz.14131. Epub 2024 Jul 25. Alzheimers Dement. 2024. PMID: 39051173 Free PMC article.
-
Probing locus coeruleus functional network in healthy aging and its association with Alzheimer's disease biomarkers using pupillometry.Alzheimers Res Ther. 2025 Feb 27;17(1):53. doi: 10.1186/s13195-025-01701-1. Alzheimers Res Ther. 2025. PMID: 40016783 Free PMC article.
-
Locus coeruleus tau validates and informs high-resolution MRI in aging and at earliest Alzheimer's pathology stages.Acta Neuropathol Commun. 2025 Feb 28;13(1):44. doi: 10.1186/s40478-025-01957-6. Acta Neuropathol Commun. 2025. PMID: 40022196 Free PMC article.
-
CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis.Lancet Neurol. 2016 Jun;15(7):673-684. doi: 10.1016/S1474-4422(16)00070-3. Epub 2016 Apr 8. Lancet Neurol. 2016. PMID: 27068280
-
Selegiline for Alzheimer's disease.Cochrane Database Syst Rev. 2003;(1):CD000442. doi: 10.1002/14651858.CD000442. Cochrane Database Syst Rev. 2003. PMID: 12535396
References
-
- Counts SE, Mufson EJ. Chapter 12-Locus coeruleus. In The Human Nervous System. 3rd ed.; Mai JK, Paxinos, G, Eds; Academic Press: San Diego, CA, USA, 2012:425‐438. doi: 10.1016/B978-0-12-374236-0.10012-4 - DOI
-
- Theofilas P, Ehrenberg AJ, Dunlop S, et al. Locus coeruleus volume and cell population changes during Alzheimer's disease progression: a stereological study in human postmortem brains with potential implication for early‐stage biomarker discovery. Alzheimers Dement. 2017;13: 236‐246. doi: 10.1016/j.jalz.2016.06.2362 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

