Salmonid sensory system development is affected by climate change driven temperature increases
- PMID: 40596713
- PMCID: PMC12217226
- DOI: 10.1038/s41598-025-99784-1
Salmonid sensory system development is affected by climate change driven temperature increases
Abstract
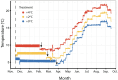
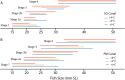
Increases in water temperature due to global climate change are known to alter the course and timing of fish development. The mechanosensory lateral line (LL) system mediates flow-sensing behaviors vital for survival in fishes, but the effects of increased water temperatures resulting from climate change on its development have not been examined. Here LL development was documented in a cold-water salmonid (brook trout, Salvelinus fontinalis) reared at the thermograph of a long-term study stream (ambient) and two higher temperatures (+ 2 and + 4 °C) that reflect projected increases within their native range. At these two higher temperatures, fish reach crucial early life history transitions earlier (e.g., hatch, "swim-up" from gravel nests into the water column) and are larger in size through the parr (juvenile) stage. Early forming canal neuromast receptor organs are larger, and the process of canal morphogenesis is also accelerated suggesting potential consequences for neuromast function and presumably for LL-mediated behaviors. A potential mismatch between the timing of transitions in early life history stages, the ability to carry out LL-mediated behaviors (e.g., prey detection), and the timing of the seasonal emergence of their preferred prey, could have serious implications for cold-water salmonid ecology and survival.
Keywords: Brook trout; Flow sensing; Lateral line; Neuromast; Ontogeny.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Development of the cranial lateral line system of Brook Trout, Salvelinus fontinalis (Teleostei: Salmonidae): Evolutionary and ecological implications.J Morphol. 2024 Aug;285(8):e21754. doi: 10.1002/jmor.21754. J Morphol. 2024. PMID: 39136647
-
The Silverjaw Minnow, Ericymba buccata: An Extraordinary Lateral Line System and its Contribution to Prey Detection.Integr Comp Biol. 2024 Sep 17;64(2):459-479. doi: 10.1093/icb/icae111. Integr Comp Biol. 2024. PMID: 38992208 Free PMC article.
-
Temperature sensitivity of the interspecific interaction strength of coastal marine fish communities.Elife. 2023 Jul 11;12:RP85795. doi: 10.7554/eLife.85795. Elife. 2023. PMID: 37431235 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
References
-
- Gauldie, R. W., Coote, G., West, I. F. & Radtke, R. L. The influence of temperature on the fluorine and calcium composition of fish scales. Tissue Cell22(5), 645–654. 10.1016/0040-8166(90)90061-D (1990). - PubMed
-
- Townsend, D. W., Radtke, R. L., Corwin, S. & Libby, D. A. Strontium: calcium ratios in juvenile Atlantic herring Clupeaharengus L. otoliths as a function of water temperature. J. Exp. Mar. Biol. Ecol.160(1), 131–140. 10.1016/0022-0981(92)90115-Q (1992).
-
- Farrell, A. P. Environment, antecedents and climate change: Lessons from the study of temperature physiology and river migration of salmonids. J. Exp. Biol.212(23), 3771–3780. 10.1242/jeb.023671 (2009). - PubMed
-
- Wedekind, C. & Kueng, C. Shift of spawning season and effects of climate warming on developmental stages of a grayling (Salmonidae). Conserv. Biol.24(5), 1418–1423. 10.1111/j.1523-1739.2010.01534.x (2010). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

