Gut microbial Nordihydroguaiaretic acid suppresses macrophage pyroptosis to regulate epithelial homeostasis and inflammation
- PMID: 40596758
- PMCID: PMC12233881
- DOI: 10.1080/19490976.2025.2518338
Gut microbial Nordihydroguaiaretic acid suppresses macrophage pyroptosis to regulate epithelial homeostasis and inflammation
Abstract
Background: Aging is associated with increased severity of inflammatory bowel disease (IBD). Gut senescence and altered environmental factors contribute to changes in the intestinal metabolome, particularly in frail older individuals. However, the role of age-associated dysbiosis, characterized by a decline in beneficial gut microbiota and their metabolites, in exacerbating IBD remains unclear.
Methods: To investigate the impact of aging-associated dysbiosis on colitis development, we employed fecal microbiota transplantation (FMT) in wild-type and IL-10-deficient mice. Aged mice were treated with gut microbiota from either young or aged mice and then subjected to dextran sulfate sodium (DSS) to induce experimental colitis. 16S rDNA sequencing and metabolomics were used to analyze microbial and metabolite profiles. Single-cell RNA sequencing (scRNA-seq) was performed to characterize lamina propria CD45+ immune cell composition.
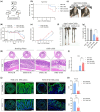
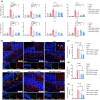
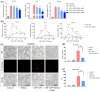
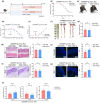
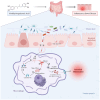
Results: Aged mice receiving microbiota from young mice exhibited less severe colitis than those receiving microbiota from aged mice, as evidenced by reduced disease activity, weight loss, and colonic shortening. Besides, aged mice displayed a significant decrease in the Lactobacillus population, accompanied by a reduction in Nordihydroguaiaretic acid (NDGA) levels. Decreased fecal NDGA levels were also observed in both IBD patients and elderly individuals. Administration of NDGA alleviated experimental colitis by downregulating the GSDMD/NR4A1/NLRP3 axis-mediated macrophage pyroptosis. Deletion of GSDMD in macrophages significantly diminished the protective effect of NDGA on colitis.
Conclusions: Our findings demonstrate that aging is associated with dysbiosis and reduced NDGA production, which increases susceptibility to intestinal inflammation. Gut microbial NDGA exhibits potential anti-inflammatory activity in colitis, suggesting a promising therapeutic target for aged-related IBD.
Keywords: Aging; IBD; NDGA; gut microbiota; macrophage pyroptosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- Jeuring SF, van den Heuvel Tr, Zeegers MP, Hameeteman WH, Romberg-Camps MJ, Oostenbrug LE, Masclee AA, Jonkers DM, Pierik MJ, van den Heuvel TRA. Epidemiology and long-term outcome of inflammatory bowel disease diagnosed at elderly age-an increasing distinct entity? Inflamm Bowel Dis. 2016;22(6):1425–1434. doi: 10.1097/MIB.0000000000000738. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
