In vitro antibacterial efficacy of a novel chicken-derived Bacillus subtilis GX15 strain and its protective mechanisms in mice challenged by Salmonella enterica serovar typhymurium
- PMID: 40596838
- PMCID: PMC12219533
- DOI: 10.1186/s12866-025-04107-z
In vitro antibacterial efficacy of a novel chicken-derived Bacillus subtilis GX15 strain and its protective mechanisms in mice challenged by Salmonella enterica serovar typhymurium
Abstract
Background: The escalating issue of bacterial resistance, coupled with stringent restrictions on antibiotic use in many countries, has prompted the search for alternative strategies to combat bacterial infections. Probiotics, such as Bacillus subtilis (B. subtilis), have been widely recognized for their ability to inhibit pathogenic bacteria proliferation and protect hosts from infection-related damage.
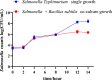
Results: In this study, a novel strain of B. subtilis, designated GX15, was isolated from the intestine of a healthy chicken. We systematically evaluated its in vitro antimicrobial activity and protective effects against Salmonella Typhimurium SM022 (S. Typhimurium SM022) infection in mice. GX15 exhibited broad-spectrum antibacterial activity, effectively inhibited the growth of both Gram-positive and Gram-negative bacteria, including S. Typhimurium SM022, Escherichia coli, and Staphylococcus aureus. In a murine model, S. Typhimurium SM022 infection induced clinical symptoms such as diarrhea, anorexia, and fur ruffling in C57BL/6 mice. Oral administration of GX15 at 10⁸ CFU/mL significantly attenuated these effects, reducing the elevation of immune organ indices, limiting bacterial translocation to peripheral tissues, and alleviating histopathological damage to the liver and intestinal tissues. Notably, GX15 did not alter antioxidant indices or immunoglobulin levels. However, it markedly modulated the expression of cytokines including GM-CSF, IL-6, TLR2, IL-10, and TGF-β in the small intestine, suggesting an immunoregulatory mechanism of action.
Conclusion: B. subtilis GX15 exhibits potent probiotic activity and represents a promising prophylactic candidate against S. Typhimurium infections. These findings provide valuable insights into the potential use of GX15 as an alternative strategy for the prevention and control of salmonellosis.
Keywords: Bacillus subtilis; Salmonella Typhimurium; Immunity; Probiotic; Protection.
© 2025. The Author(s).
Conflict of interest statement
Ethics approval and consent to participate: All experimental protocols were approved by Guangxi Department of Science and Technology. All animal procedures were performed in accordance with the protocols approved by the Institutional Animal Experimental Ethical Inspection Form of Guangxi Veterinary Research Institute, Nanning, China(8/2014/JU). The ethic number is No. 202301016. The study was carried out in compliance with the ARRIVE guidelines. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



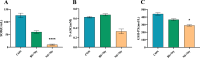



Similar articles
-
A multispecies bacterial-based direct-fed microbial alleviates Salmonella invasion and supports in vitro epithelial integrity.J Anim Sci. 2024 Jan 3;102:skae304. doi: 10.1093/jas/skae304. J Anim Sci. 2024. PMID: 39383437
-
Optimistic effects of dual nano-encapsulated probiotics on breeders laying performance, intestinal barrier functions, immunity and resistance against Salmonella Typhimurium challenge.Sci Rep. 2025 Jul 30;15(1):27836. doi: 10.1038/s41598-025-05495-y. Sci Rep. 2025. PMID: 40738913 Free PMC article.
-
IITR00803: a benzoxazole-nitrothiophene small molecule with broad-spectrum antibacterial potential.Microbiol Spectr. 2025 Sep 2;13(9):e0114425. doi: 10.1128/spectrum.01144-25. Epub 2025 Aug 12. Microbiol Spectr. 2025. PMID: 40792514 Free PMC article.
-
Probiotics for the prevention of pediatric antibiotic-associated diarrhea.Cochrane Database Syst Rev. 2011 Nov 9;(11):CD004827. doi: 10.1002/14651858.CD004827.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 Dec 22;(12):CD004827. doi: 10.1002/14651858.CD004827.pub4. PMID: 22071814 Updated.
-
Prophylactic antibiotics for preventing gram-positive infections associated with long-term central venous catheters in adults and children receiving treatment for cancer.Cochrane Database Syst Rev. 2021 Oct 7;10(10):CD003295. doi: 10.1002/14651858.CD003295.pub4. Cochrane Database Syst Rev. 2021. PMID: 34617602 Free PMC article.
References
-
- Friedman ND, Temkin E, Carmeli Y. The negative impact of antibiotic resistance. Clin Microbiol Infect. 2016;22(5):416–22. - PubMed
-
- Casewell M, Friis C, Marco E, McMullin P, Phillips I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J Antimicrob Chemother. 2003;52(2):159–61. - PubMed
-
- Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–98. - PubMed
MeSH terms
Substances
Grants and funding
- 240108/Laibin Capacity and Condition Building for Science and Technology Innovation
- nycytxgxcxtd20210905/Guangxi Innovation Team Construction Project of National Modern Agricultural Industry Technology System
- 20201Y075/Guizhou Science and Technology Program
- AB241484045/Guangxi Key Research and Development Program
- 220819/Laibin Key Research and Development Programme
LinkOut - more resources
Full Text Sources
Medical

