Gastric cancer risk after Helicobacter pylori eradication in gastritis and peptic ulcer: a retrospective cohort study in Japan
- PMID: 40596880
- PMCID: PMC12211893
- DOI: 10.1186/s12876-025-04034-3
Gastric cancer risk after Helicobacter pylori eradication in gastritis and peptic ulcer: a retrospective cohort study in Japan
Abstract
Background: Helicobacter pylori infection is an important risk factor for gastric cancer. In Japan, national health insurance has covered eradication therapy for H. pylori infection-associated gastritis from 2013. However, gastric cancer was the fourth leading cause of cancer death in 2023. We aimed to investigate differences in gastric cancer risk among patients with gastritis, gastric ulcer, duodenal ulcer, and gastric ulcer and duodenal ulcer after H. pylori eradication.
Methods: This retrospective cohort study used the JMDC Claims Database from February 21, 2013, to August 31, 2023. Patients who received first-line H. pylori eradication therapy and were diagnosed with H. pylori-associated gastritis, gastric ulcer, or duodenal ulcer in the same month or the month before the first eradication therapy prescription were included. Two antibacterial drugs and an acid secretion inhibitor or triple-drug blister-packaged product were prescribed. The primary outcome was gastric cancer incidence. A Cox proportional hazards regression analysis was used to estimate hazard ratios (HRs). A propensity score approach was used to minimize the effect of confounding measures.
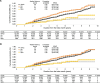
Results: Of 17,245,330 beneficiaries, 148,489 were included. In the weighted cohort (after propensity matching), statistically significant differences were observed in HRs between H. pylori-associated gastritis and duodenal ulcer (HR using the latter as a reference [95% confidence interval]: 2.03 [1.31-3.13]; p = 0.001), and between gastric ulcer and duodenal ulcer (2.37 [1.52-3.71]; p < 0.001). The cumulative probabilities (95% confidence interval) per the median follow-up years (3.8 years for all) were 0.44% (0.39-0.48) for H. pylori-associated gastritis, 0.54% (0.46-0.63) for gastric ulcer, 0.22% (0.10-0.33) for duodenal ulcer, and 0.26% (0.08-0.50) for gastric ulcer and duodenal ulcer.
Conclusions: Patients with H. pylori-associated gastritis and gastric ulcer had a higher risk of gastric cancer than patients with duodenal ulcer, indicating that gastric atrophy remains a risk factor after H. pylori eradication therapy. Careful monitoring, such as by endoscopic examination, is required after successful eradication of H. pylori in patients at higher risk.
Keywords: Claims database; Duodenal ulcer; Eradication therapy; Gastric atrophy; Gastric cancer; Gastric ulcer; Gastritis; Helicobacter pylori.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This was a retrospective study using data from a claims database. Because of the anonymous nature of the analysis and the absence of direct patient involvement, ethical approval and informed patient consent were not required, based on the Ethical Guidelines for Epidemiological Research issued by the Japanese Ministry of Health, Labour and Welfare. Consent for publication: Not applicable. Competing interests: KS has received lecture fees from Biofermin Pharmaceutical Co. Ltd., Fujifilm Medical Co. Ltd., and Takeda Pharmaceutical Company Limited, and consultant fees for participation on a data safety monitoring board or advisory board from Fujifilm Medical Co. Ltd. CS is an employee of Takeda Pharmaceutical Company Limited. MO and RI were employees of Takeda Pharmaceutical Company Limited at the time the study was conducted. No other disclosures were reported.
Figures


References
-
- Kato M, Ota H, Okuda M, Kikuchi S, Satoh K, Shimoyama T, Suzuki H, Handa O, Furuta T, Mabe K et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 revised edition. Helicobacter 2019, 24(4):e12597. - PubMed
-
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345(11):784–9. - PubMed
-
- IARC Helicobacter pylori Working Group. Helicobacter pylori eradication as a strategy for preventing gastric cancer (IARC Working Group Report Volume 8). Lyon, France: International Agency for Research on Cancer; 2014.
-
- Health, Labour and Welfare Statistics Association. 国民衛生の動向 2024/2025. J Health Welfare Stat. 2024;71(9):1–412.
-
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52(24):6735–40. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

