Efficacy and safety of inhaled ambroxol hydrochloride solution in patients with lower respiratory tract infectious diseases: a randomized, double-blind, placebo-controlled, multicentre clinical trial
- PMID: 40596900
- PMCID: PMC12211132
- DOI: 10.1186/s12879-025-11194-w
Efficacy and safety of inhaled ambroxol hydrochloride solution in patients with lower respiratory tract infectious diseases: a randomized, double-blind, placebo-controlled, multicentre clinical trial
Abstract
Background: Ambroxol is a widely used mucoactive agent, but the efficacy of inhaled ambroxol in patients with lower respiratory tract infectious (LRTI) disease is poorly understood. This trial aimed to compare the efficacy and safety of inhaled ambroxol with those of placebo in patients with LRTI diseases.
Methods: In this randomized, double-blind, placebo-controlled, multicentre clinical trial, 240 patients with LRTI diseases were randomized to receive inhaled ambroxol hydrochloride solution (ambroxol group, N = 120) or placebo (placebo group, N = 120) twice daily for 7 days.
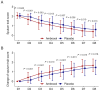
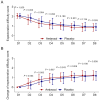
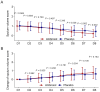
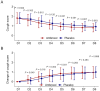
Results: Compared with the placebo group, the ambroxol group had lower sputum trait scores and greater changes in sputum trait scores from Day 2 to Day 8. Compared with the placebo group, the ambroxol group presented lower expectoration difficulty scores and greater changes in expectoration difficulty scores on Days 2, 3, and 6. The sputum volume scores on Days 6, 7, and 8 were lower in the ambroxol group than in the placebo group, but the change in the sputum volume score was not different between the groups. Compared with the placebo group, the ambroxol group had lower cough scores on Days 3, 5, 6, and 7, as well as greater changes in cough scores on Days 2, 3, and 5. The incidences of adverse events (10.8% versus 6.7%), serious adverse events (0.8% versus 0.0%), and adverse reactions (4.2% versus 3.3%) were not different between the ambroxol group and the placebo group.
Conclusions: Inhaled ambroxol is better at ameliorating respiratory symptoms and has comparable safety to placebo in patients with LRTI diseases.
Keywords: Ambroxol; Efficacy; Inhalation; Lower respiratory tract infectious diseases; Safety.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Patients signed the informed consent. The Ethics Committee of Peking University People’s Hospital approved the trial with the Ethics number of 2013-21. The trial was registered on Chinese Clinical Trial Registry with the number of ChiCTR2300072467. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Salluh JIF, Povoa P, Beane A, Kalil A, Sendagire C, Sweeney DA, Pilcher D, Polverino E, Tacconelli E, Estenssoro E, Frat JP, Ramirez J, Reyes LF, Roca O, Nseir S, Nobre V, Lisboa T, Martin-Loeches I. Challenges for a broad international implementation of the current severe community-acquired pneumonia guidelines. Intensive Care Med. 2024;50(4):526–38. 10.1007/s00134-024-07381-z - DOI - PubMed
-
- Introduction to the. Database efficacy of different treatment modalities for lower respiratory tract infections in children. Pediatric Discovery Online.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

