Infections and mortality in ICU patients undergoing continuous renal replacement therapy: a retrospective cohort study
- PMID: 40596911
- PMCID: PMC12220524
- DOI: 10.1186/s12882-025-04272-3
Infections and mortality in ICU patients undergoing continuous renal replacement therapy: a retrospective cohort study
Abstract
Background: Critically ill patients receiving continuous renal replacement therapy (CRRT) are at increased risk for multidrug-resistant infections and infection-related mortality. Altered pharmacokinetics in CRRT may contribute to inadequate antimicrobial exposure and therapeutic failure. However, limited data exist on infection burden and resistance patterns specific to this population.
Methods: We conducted a retrospective cohort study of ICU patients receiving continuous venovenous hemodialysis (CVVHD) at a tertiary academic center between May 2016 and April 2020. Patients were included if they received CRRT for ≥ 48 h, had at least one positive microbial culture, and received at least one antimicrobial of interest. Data were collected on infection sources, pathogens, resistance patterns, and mortality.
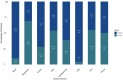
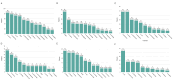
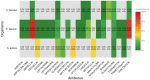
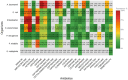
Results: Among 661 CRRT recipients, 394 (59.6%) had at least one positive culture. The most common infection sites were respiratory (69.0%), skin and soft tissue (53.8%), and intra-abdominal (38.8%). Intra-abdominal and bloodstream infections had the highest mortality (63.7% and 57.7%, respectively). Vancomycin-resistant E. faecium (83.3%), cefepime-resistant A. baumannii (100%), and P. aeruginosa with high β-lactam resistance were prominent. These resistance profiles diverged from general ICU trends.
Conclusion: ICU patients receiving CRRT experience high rates of multidrug-resistant infections and associated mortality. Tailored dosing strategies, including dual empiric coverage in select cases, and CRRT-specific antimicrobial stewardship are essential to improve outcomes in this high-risk population.
Keywords: Antimicrobial resistance; Continuous renal replacement therapy; Critical care; Infection-related mortality.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Human ethics and consent to participate: This study was approved by the Institutional Review Board at the University of Chicago and conducted in accordance with institutional and national research ethics guidelines. As a retrospective chart review using anonymized data, the requirement for informed consent was waived by the IRB. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Badel JC, Garcia LA, Soto-Doria MJ, Musso CG. Dialysis prescription in acute kidney injury: when and how much? Int Urol Nephrol. 2021;53(3):489–96. - PubMed
-
- Charoensareerat T, Chaijamorn W, Kerdnimith P, Kosumwisaisakul N, Teeranaew P, Rungkitwattanakul D, Boonpeng A, Srisawat N, Pattharachayakul S. Optimal meropenem dosing regimens in patients undergoing continuous renal replacement therapy: systematic review and Monte Carlo simulations. Blood Purif. 2023;52(6):503–15. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

