Motivation toward physical activity and nutrition in older cancer patients: the MONAGE protocol using ecological momentary assessment and accelerometers
- PMID: 40596930
- PMCID: PMC12220263
- DOI: 10.1186/s12877-025-06130-1
Motivation toward physical activity and nutrition in older cancer patients: the MONAGE protocol using ecological momentary assessment and accelerometers
Abstract
Background: Older adults with cancer struggle to maintain recommended levels of physical activity and nutrition during treatments. Collecting data in a real-life context provides a better understanding of the motivational and behavioral dynamics of this population, which is underrepresented in clinical trials. Ecological momentary assessment (EMA) is a frequently used approach to collect repeated measures in real time in a naturalistic environment. This approach can provide insights into the temporal dynamics of psychological processes, such as motivation toward health behaviors. The aim of this study is to identify clusters based on predictive motivational variables related to physical activity and nutritional behaviors among older patients with cancer, via repeated measures.
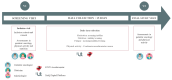
Methods: In this project, older patients with a cancer (≥ 70 years old) complete physical capacity assessments, malnutrition identification, and a comprehensive geriatric assessment with G-code. After that, participants engage in a 2-week data collection protocol combining EMA and sensor-based monitoring of behavior. The participants wear an ActiGraph GT3X-BT accelerometer (ActiGraph, LLC) on their nondominant waist to measure physical activity. EMA questionnaires are delivered 3 times per day - morning (between 8am and 10am), midday (between 12pm and 2pm) and evening (between 7pm and 8pm) - via smartphones or computers with the UniQ digital application. Motivational constructs based on the Theory of Planned Behavior are collected in the morning and at midday. Nutritional behavior data are collected at midday and in the evening. Fatigue is collected in the morning, at midday and in the evening. The data analysis strategy shall include Multilevel Vector Autoregressive Models with k-means clustering.
Discussion: This project seeks to identify clusters in older patients with cancer based on key motivational predictors related to physical activity and nutritional behaviors, via approaches for collecting repeated measurements in real time within a naturalistic environment. This research into the mechanisms of action between motivation and behaviors could optimize care pathways by designing appropriate intervention content, under conditions where the content will be most effective, to promote sustained behavior change among older adults with cancer.
Trial registration: ClinicalTrials.gov (NCT06445140). Registered on 06 June 2024.
Supplementary Information: The online version contains supplementary material available at 10.1186/s12877-025-06130-1.
Keywords: Accelerometers; Aging; Behavior change; Cancer; Care pathways; Ecological momentary assessment; Exercise; Motivation; Nutrition; Times series data.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This research project has been approved by the national ethics committee (Comité des Personnes Nord Ouest III, accepted on 30 May 2024) and the ANSM (French National Agency Authority for the Safety of Health Products) has been informed (19 June 2024). This research will be conducted in accordance with the French Public Health Code, the Good Clinical Practice and the Declaration of Helsinki. This study has been registered on clinicaltrial.gov (NCT06445140), 06 June 2024. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Characterising physical activity patterns in community-dwelling older adults using digital phenotyping: a 2-week observational study protocol.BMJ Open. 2025 May 24;15(5):e095769. doi: 10.1136/bmjopen-2024-095769. BMJ Open. 2025. PMID: 40413040 Free PMC article.
-
Associations Between Social Cognitive Determinants and Movement-Related Behaviors in Studies Using Ecological Momentary Assessment Methods: Systematic Review.JMIR Mhealth Uhealth. 2023 Apr 7;11:e44104. doi: 10.2196/44104. JMIR Mhealth Uhealth. 2023. PMID: 37027185 Free PMC article.
-
Use of Electronic Ecological Momentary Assessment Methodologies in Physical Activity, Sedentary Behavior, and Sleep Research in Young Adults: Systematic Review.J Med Internet Res. 2023 Jun 29;25:e46783. doi: 10.2196/46783. J Med Internet Res. 2023. PMID: 37384367 Free PMC article.
-
Smartphone-Delivered Ecological Momentary Interventions Based on Ecological Momentary Assessments to Promote Health Behaviors: Systematic Review and Adapted Checklist for Reporting Ecological Momentary Assessment and Intervention Studies.JMIR Mhealth Uhealth. 2021 Nov 19;9(11):e22890. doi: 10.2196/22890. JMIR Mhealth Uhealth. 2021. PMID: 34806995 Free PMC article.
-
A Systematic Review of Methods and Procedures Used in Ecological Momentary Assessments of Diet and Physical Activity Research in Youth: An Adapted STROBE Checklist for Reporting EMA Studies (CREMAS).J Med Internet Res. 2016 Jun 21;18(6):e151. doi: 10.2196/jmir.4954. J Med Internet Res. 2016. PMID: 27328833 Free PMC article.
References
-
- Causes de décès des. personnes âgées | Insee [Internet]. [cited 2025 Feb 19]. Available from: https://www.insee.fr/fr/statistiques/2386247#figure1_radio2
-
- Schwartz A, Mere P, Subtil F, Labrosse H, Farsi F, Guittard L, et al. RCP dédiée à l’onco-gériatrie: décisions et suivi à quatre Mois. Bull Cancer (Paris). 2022;109(6):659–69. - PubMed
-
- Paillaud E, Soubeyran P, Caillet P, Cudennec T, Brain E, Terret C, et al. Multidisciplinary development of the geriatric core dataset for clinical research in older patients with cancer: A French initiative with international survey. Eur J Cancer. 2018;103:61–8. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials