BAIAP2 as a driver of tumor progression in urothelial bladder cancer
- PMID: 40596964
- PMCID: PMC12211370
- DOI: 10.1186/s12885-025-14470-9
BAIAP2 as a driver of tumor progression in urothelial bladder cancer
Abstract
Background: Urothelial bladder cancer (UBC) is a highly heterogeneous malignancy with poor prognosis in muscle-invasive and high-grade subtypes. Epithelial-mesenchymal transition (EMT) drives tumor aggressiveness, yet its molecular mechanisms in UBC remain unclear. BAI1 associated protein 2 (BAIAP2) has been linked to cancer progression but remains unexplored in UBC. This study investigates the expression, functional role, and regulatory mechanisms of BAIAP2 in UBC, focusing on its contribution to tumor aggressiveness.
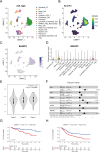
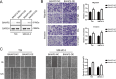
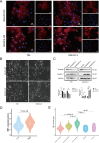
Methods: This study investigated the role of BAIAP2 in UBC using single-cell data analysis, bioinformatics, and functional assays. BAIAP2 expression was analyzed across UBC sub-populations, stages, and molecular subtypes via immunohistochemistry and quantitative methods. Transwell migration, invasion, and wound-healing assays were used to assess the impact of BAIAP2 knockdown and overexpression on cell behavior. EMT-like changes were examined through immunofluorescence and bright-field imaging. The roles of BAIAP2 in regulation of EMT pathways and its interaction with the transcription factor RELA were validated by Western blot analysis. Enrichment analysis of TCGA-BLCA datasets identified associated gene ontology terms and KEGG pathways.
Results: BAIAP2 was overexpressed in UBC, particularly in muscle-invasive and high-grade subtypes, and correlated with poor prognosis. Functional assays showed BAIAP2 promoted migration, invasion, and EMT-like changes, while its knockdown suppressed these behaviors. Bioinformatics analysis linked BAIAP2 to the transcription factor RELA, with RELA knockdown reducing BAIAP2 expression. Enrichment analysis implicated BAIAP2 in cytoskeletal reorganization and tumor progression, highlighting its role in UBC aggressiveness and potential for further therapeutic investigation.
Conclusions: BAIAP2 was highly expressed in muscle-invasive and high-grade tumors and was associated with poor prognosis. It promoted metastasis and EMT through activation of cytoskeletal remodeling. These findings identified BAIAP2 as a promising biomarker and a potential therapeutic target for the aggressive UBC.
Keywords: BAI1 associated protein 2 (BAIAP2); Epithelial-mesenchymal transition (EMT); Progression; Urothelial bladder cancer (UBC).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The study was approved by the Ethical Committee of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (Approval No. 2021-K-101-01). Written informed consent was obtained from all participating patients. The consent process ensured that patients were fully informed about the study’s purpose, procedures, potential risks, and benefits, in accordance with the principles of the Declaration of Helsinki. All patient data were anonymized to protect confidentiality. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

