Ex vivo engineering of phagocytic signals in breast cancer cells for a whole tumor cell-based vaccine
- PMID: 40596971
- PMCID: PMC12211345
- DOI: 10.1186/s12885-025-14432-1
Ex vivo engineering of phagocytic signals in breast cancer cells for a whole tumor cell-based vaccine
Abstract
Background: Today, cell therapies are constantly evolving and providing new options for cancer patients. These therapies are mostly based on the inoculation of immune cells extracted from a person's own tumor; however, some studies using whole tumor cell-based vaccines are approaching the level of maturity required for clinical use. Although these latest therapies will have to be developed further and adapted to overcome many ethical barriers, there is no doubt that therapeutic cancer vaccines are the next frontier of immunotherapy.
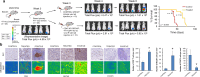
Methods: Ionizing radiation and CD47 knockout via CRISPR-Cas9 genome editing were used to optimize the macrophage-mediated phagocytosis of breast cancer cells. These cells were subsequently used in several mouse models to determine their potential as novel whole-cell-based vaccines to drive antitumor immunity. To improve the recognition of tumor cells by activated immune cells, this cellular therapy was combined with anti-PD-1 antibody treatments.
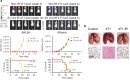
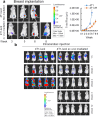
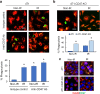
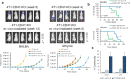
Results: Here, we showed that irradiation of 4T1 breast cancer cells increases their immunogenicity and, when injected into the blood of immunocompetent mice, elicits a complete antitumor immune response mediated, in part, by the adaptive immune system. Next, to improve the macrophage-mediated phagocytosis of breast cancer cells, we knocked out CD47 in 4T1 cells. When injected in the bloodstream, irradiated CD47 knockout cells activated both the adaptive and the innate immune systems. Therefore, we used these ex vivo engineered cells as a whole tumor cell-based vaccine to treat breast tumors in immunocompetent mice. A better response was obtained when these cells were combined with an anti-PD-1 antibody.
Conclusion: These results suggest that tumor cells obtained from surgical samples of a breast cancer patient could be engineered ex vivo and used as a novel cell therapy to drive antitumor immunity.
Keywords: Anti-tumor immunity; Breast cancer; CD47; Ionizing radiation; Whole tumor cell-based vaccines.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Animals were bred and maintained according to Spanish legislation on the ‘Protection of Animals used for Experimental and other Scientific Purposes’ and in accordance with the directives of the European Community. All animal procedures were approved by the Ethical Committee of the University of Murcia and the Direccion General de Ganaderia y Pesca, Comunidad Autonoma de Murcia (Project reference A13151101). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- 21407/FPI/20/Fundación Séneca
- FSRM/10.13039/100007801(22544/PI/24)/Fundación Séneca
- FSRM/10.13039/100007801(22544/PI/24)/Fundación Séneca
- FSRM/10.13039/100007801(22544/PI/24)/Fundación Séneca
- FSRM/10.13039/100007801(22544/PI/24)/Fundación Séneca
- FSRM/10.13039/100007801(22544/PI/24)/Fundación Séneca
- PID2023-149281OB-I00/Ministerio de Ciencia, Innovación y Universidades
- CPP2023-010510/Ministerio de Ciencia, Innovación y Universidades
- PID2023-149281OB-I00/Ministerio de Ciencia, Innovación y Universidades
- CPP2023-010510/Ministerio de Ciencia, Innovación y Universidades
- PID2023-149281OB-I00/Ministerio de Ciencia, Innovación y Universidades
- PID2023-149281OB-I00/Ministerio de Ciencia, Innovación y Universidades
LinkOut - more resources
Full Text Sources
Medical
Research Materials

