Glymphatic and neurofluidic dysfunction in classical trigeminal neuralgia: a multimodal MRI study of brain-CSF functional and structural dynamics
- PMID: 40596979
- PMCID: PMC12211343
- DOI: 10.1186/s12880-025-01801-2
Glymphatic and neurofluidic dysfunction in classical trigeminal neuralgia: a multimodal MRI study of brain-CSF functional and structural dynamics
Abstract
Objective: To investigate whether dysfunction of the glymphatic system and altered neurofluidic dynamics contribute to the pathophysiology of classical trigeminal neuralgia (CTN), and to explore the potential interplay between brain-CSF coupling and structural brain changes.
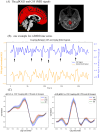
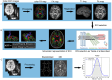
Methods: A total of 131 patients with CTN and 106 age- and sex-matched healthy controls were recruited. All participants underwent multimodal MRI, including high-resolution structural imaging, resting-state functional MRI, and diffusion tensor imaging. Key indices included choroid plexus (CP) volume as a proxy for CSF production, global BOLD-CSF coupling as a measure of functional neurofluidic interaction, and the DTI-based ALPS index reflecting glymphatic clearance. Additional markers included peak width of skeletonized mean diffusivity (PSMD) and global gray/white matter and CSF volume. Partial correlation analyses were performed between imaging metrics and clinical assessments.
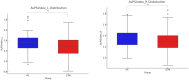
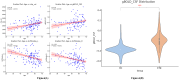
Results: CTN patients showed significantly increased CP volume (P = 0.022) and gBOLD-CSF coupling (P < 0.001), along with reduced bilateral ALPS indices (P = 0.002, P = 0.004). PSMD and CSF volume were elevated (P < 0.001, P < 0.001), while gray and white matter volumes were reduced (P = 0.028, P = 0.009). gBOLD-CSF coupling correlated positively with depression, anxiety, and pain-related disability scores (P < 0.001), and negatively with MMSE (P = 0.022).
Conclusion: This study provides multimodal MRI evidence of glymphatic dysfunction and neurofluidic alterations in CTN, supporting a conceptual framework in which disrupted brain-CSF interaction may influence peripheral sensory modulation through a putative brain-CSF-ganglion pathway. These results may inform mechanistic hypotheses and guide future research on the neurofluidic underpinnings of neuropathic pain, potentially providing new insights into the pathogenesis of CTN.
Keywords: Brain-CSF coupling; Choroid plexus volume; Classical trigeminal neuralgia; Glymphatic system; Neurofluidic dynamics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study was approved by the local ethics committee of the Affiliated Hangzhou First People’s Hospital, Westlake University School of Medicine (IRB# NO.202107002). All investigations were carried out following the Declaration of Helsinki, and all the participants provided written informed consent. Consent for publication: This study has obtained informed consents from patients, all human research procedures followed the committee’s ethical standards for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Glymphatic dysfunction in classical trigeminal neuralgia: DTI-ALPS index stratification from healthy controls to post-MVD.J Headache Pain. 2025 Jun 23;26(1):148. doi: 10.1186/s10194-025-02064-6. J Headache Pain. 2025. PMID: 40551099 Free PMC article.
-
Multimodal MRI reveals impaired glymphatic function with choroid plexus enlargement and cerebrospinal fluid expansion in alzheimer's disease.Sci Rep. 2025 Aug 19;15(1):30409. doi: 10.1038/s41598-025-15923-8. Sci Rep. 2025. PMID: 40830653 Free PMC article.
-
Alterations in the Glymphatic System and Association with Brain Structure and Cognitive Function in Moyamoya Disease.Transl Stroke Res. 2025 Aug;16(4):1173-1184. doi: 10.1007/s12975-024-01296-z. Epub 2024 Sep 9. Transl Stroke Res. 2025. PMID: 39245689
-
Structural brain alterations and changes in resting-state functional connectivity in patients with trigeminal neuralgia: A meta-analysis.Neuroimage Clin. 2025;46:103759. doi: 10.1016/j.nicl.2025.103759. Epub 2025 Feb 27. Neuroimage Clin. 2025. PMID: 40086208 Free PMC article. Review.
-
Translating state-of-the-art spinal cord MRI techniques to clinical use: A systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI.Neuroimage Clin. 2015 Dec 4;10:192-238. doi: 10.1016/j.nicl.2015.11.019. eCollection 2016. Neuroimage Clin. 2015. PMID: 26862478 Free PMC article.
References
-
- Cruccu G, Di Stefano G, Truini A. Trigeminal neuralgia. N Engl J Med. 2020;383(8):754–62. - PubMed
-
- Finnerup NB. Trigeminal neuralgia and the merit of small clinical trials. Lancet Neurol. 2022;21(11):951–3. - PubMed
-
- Jay GW, Barkin RL. Trigeminal neuralgia and persistent idiopathic facial pain (atypical facial pain). Dis Mon. 2022;68(6):101302. - PubMed
MeSH terms
Grants and funding
- 2024ZL749/Zhejiang Provincial Traditional Chinese Medicine Science and Technology
- A20231055/the Medical and Health Technology Project of Hangzhou
- Y22H185692/Natural Science Foundation of Zhejiang Province,China
- 2025KY1058/the Zhejiang Provincial Medical and Health Technology Project
- LQN25H090010/the Natural Science Foundation of Zhejiang Province
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

