Attention-driven hybrid deep learning and SVM model for early Alzheimer's diagnosis using neuroimaging fusion
- PMID: 40597079
- PMCID: PMC12210430
- DOI: 10.1186/s12911-025-03073-w
Attention-driven hybrid deep learning and SVM model for early Alzheimer's diagnosis using neuroimaging fusion
Abstract
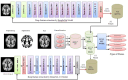
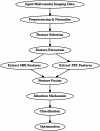
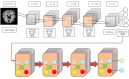
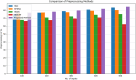
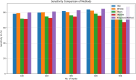
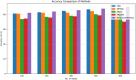
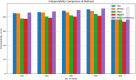
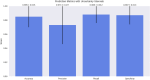
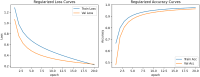
Alzheimer's Disease (AD) poses a significant global health challenge, necessitating early and accurate diagnosis to enable timely interventions. AD is a progressive neurodegenerative disorder that affects millions worldwide and is one of the leading causes of cognitive impairment in older adults. Early diagnosis is critical for enabling effective treatment strategies, slowing disease progression, and improving the quality of life for patients. Existing diagnostic methods often struggle with limited sensitivity, overfitting, and reduced reliability due to inadequate feature extraction, imbalanced datasets, and suboptimal model architectures. This study addresses these gaps by introducing an innovative methodology that combines SVM with Deep Learning (DL) to improve the classification performance of AD. Deep learning models extract high-level imaging features which are then concatenated with SVM kernels in a late-fusion ensemble. This hybrid design leverages deep representations for pattern recognition and SVM's robustness on small sample sets. This study provides a necessary tool for early-stage identification of possible cases, so enhancing the management and treatment options. This is attained by precisely classifying the disease from neuroimaging data. The approach integrates advanced data pre-processing, dynamic feature optimization, and attention-driven learning mechanisms to enhance interpretability and robustness. The research leverages a dataset of MRI and PET imaging, integrating novel fusion techniques to extract key biomarkers indicative of cognitive decline. Unlike prior approaches, this method effectively mitigates the challenges of data sparsity and dimensionality reduction while improving generalization across diverse datasets. Comparative analysis highlights a 15% improvement in accuracy, a 12% reduction in false positives, and a 10% increase in F1-score against state-of-the-art models such as HNC and MFNNC. The proposed method significantly outperforms existing techniques across metrics like accuracy, sensitivity, specificity, and computational efficiency, achieving an overall accuracy of 98.5%.
Keywords: Alzheimer's disease (AD); Classification accuracy; Disease detection; Machine learning; Neuroimaging; Recursive feature elimination.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Predicting cognitive decline: Deep-learning reveals subtle brain changes in pre-MCI stage.J Prev Alzheimers Dis. 2025 May;12(5):100079. doi: 10.1016/j.tjpad.2025.100079. Epub 2025 Feb 6. J Prev Alzheimers Dis. 2025. PMID: 39920001 Free PMC article.
-
GAN-enhanced deep learning for improved Alzheimer's disease classification and longitudinal brain change analysis.Front Med (Lausanne). 2025 Jun 17;12:1587026. doi: 10.3389/fmed.2025.1587026. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40600033 Free PMC article.
-
Enhanced particle swarm optimization for feature selection in SVM-based Alzheimer's disease diagnosis.Sci Rep. 2025 Jul 7;15(1):24243. doi: 10.1038/s41598-025-03270-7. Sci Rep. 2025. PMID: 40624099 Free PMC article.
-
Advancing respiratory disease diagnosis: A deep learning and vision transformer-based approach with a novel X-ray dataset.Comput Biol Med. 2025 Aug;194:110501. doi: 10.1016/j.compbiomed.2025.110501. Epub 2025 Jun 9. Comput Biol Med. 2025. PMID: 40494170
-
A systematic review: Brain age gap as a promising early diagnostic biomarker for Alzheimer's disease.J Neurol Sci. 2025 Aug 15;475:123563. doi: 10.1016/j.jns.2025.123563. Epub 2025 Jun 3. J Neurol Sci. 2025. PMID: 40494037 Review.
References
-
- Hassan E, Saber A, Elbedwehy S. Knowledge distillation model for acute lymphoblastic leukemia detection: exploring the impact of nesterov-accelerated adaptive moment Estimation optimizer. Biomed Signal Process Control. 2024;94:106246.
-
- Saber A, et al. An optimized ensemble model based on meta-heuristic algorithms for effective detection and classification of breast tumors. Neural Comput Appl. 2025;37(6):4881–94.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical