ATF3-mediated inhibition of Trem2 by Toxoplasma gondii contributes to adverse pregnancy outcomes
- PMID: 40597375
- PMCID: PMC12210541
- DOI: 10.1186/s13071-025-06894-w
ATF3-mediated inhibition of Trem2 by Toxoplasma gondii contributes to adverse pregnancy outcomes
Abstract
Background: Approximately one in three people worldwide have been exposed to Toxoplasma gondii (T. gondii). Primary infection with T. gondii during pregnancy can cause severe complications. Our previous study demonstrated that deficiency of triggering receptor expressed on myeloid cells 2 (Trem2) exacerbates pregnancy-related complications in T. gondii-infected mice. However, understanding the mechanisms by which T. gondii modulates Trem2 expression in macrophages remains an unmet challenge.
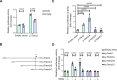
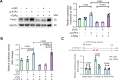
Methods: A mouse pregnancy model of T. gondii infection and an in vitro cellular stimulation model using soluble T. gondii antigens (sTgAg) were used to assess Trem2 expression. Recombinant plasmids containing the full-length Trem2 promoter were constructed to evaluate the effect of sTgAg on promoter activity, followed by the construction of truncated promoter plasmids to identify key regulatory regions. Transcription factors potentially binding to the Trem2 promoter were predicted using PROMO and JASPAR, with ATF3 identified as responsive to sTgAg stimulation via western blot analysis. The binding of ATF3 to the Trem2 promoter was validated by chromatin immunoprecipitation (ChIP) assays. Finally, ATF3 knockdown experiments were performed to determine its role in mediating the inhibitory effect of sTgAg on Trem2 expression.
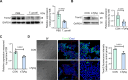
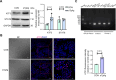
Results: T. gondii significantly suppressed Trem2 expression in both mouse placentas and cellular models, with truncated promoter assays identifying key regulatory regions of the Trem2 promoter inhibited by sTgAg. ATF3 was identified as a transcription factor responsive to sTgAg stimulation, which bound to the Trem2 promoter. Importantly, knockdown of ATF3 restored Trem2 expression, demonstrating its critical role in mediating the inhibitory effect of sTgAg.
Conclusions: We identified that sTgAg may target and inhibit Trem2 expression through the transcription factor ATF3, and inhibition of ATF3 activity may help maintain Trem2 expression in macrophages, providing a potential therapeutic approach to avert negative effects on pregnancy related to T. gondii infection.
Keywords: ATF3; Toxoplasma gondii; Trem2 promoter; Adverse pregnancy outcomes; Macrophages.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Every study utilizing mice was executed in full compliance with the Guidelines for the Management and Use of Laboratory Animals (Science Press of China, 2016). These procedures used in the research received approval from the Animal Care and Use Committee of Nantong University (approval number P20230302-013). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Knockdown of trem2 promotes proinflammatory microglia and inhibits glioma progression via the JAK2/STAT3 and NF-κB pathways.Cell Commun Signal. 2024 May 15;22(1):272. doi: 10.1186/s12964-024-01642-6. Cell Commun Signal. 2024. PMID: 38750472 Free PMC article.
-
Trem2/Syk/PI3K axis contributes to the host protection against Toxoplasma gondii-induced adverse pregnancy outcomes via modulating decidual macrophages.PLoS Pathog. 2024 Sep 9;20(9):e1012543. doi: 10.1371/journal.ppat.1012543. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39250507 Free PMC article.
-
Endoplasmic Reticulum Stress-Induced triggering Receptor Expressed on Myeloid Cells 2 (TREM2) Downregulation Exacerbates Platelet Activation and Myocardial Infarction in Patients With Coronary Artery Disease.J Am Heart Assoc. 2025 Jul;14(13):e041220. doi: 10.1161/JAHA.124.041220. Epub 2025 Jun 23. J Am Heart Assoc. 2025. PMID: 40551335
-
Seroprevalence of Toxoplasma gondii in the Iranian pregnant women: A systematic review and meta-analysis.Acta Trop. 2016 Jun;158:160-169. doi: 10.1016/j.actatropica.2016.03.003. Epub 2016 Mar 4. Acta Trop. 2016. PMID: 26952970
-
A systematic review and meta-analysis of Toxoplasma gondii infection among the Mexican population.Parasit Vectors. 2012 Nov 26;5:271. doi: 10.1186/1756-3305-5-271. Parasit Vectors. 2012. PMID: 23181616 Free PMC article.
References
-
- Cao J, Jiang L, Miller LH. Decoding infection and transmission: deciphering the mystery of infectious diseases from data-based research. Decod Infect Transm. 2023;1:100001.
-
- Smith NC, Goulart C, Hayward JA, Kupz A, Miller CM, van Dooren GG. Control of human toxoplasmosis. Int J Parasitol. 2021;51:95–121. - PubMed
-
- Harker KS, Ueno N, Lodoen MB. Toxoplasmagondii dissemination: a parasite’s journey through the infected host. Parasite Immunol. 2015;37:141–9. - PubMed
-
- Gómez-Chávez F, Murrieta-Coxca JM, Caballero-Ortega H, Morales-Prieto DM, Markert UR. Host-pathogen interactions mediated by extracellular vesicles in Toxoplasmagondii infection during pregnancy. J Reprod Immunol. 2023;158:103957. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

