Shikonin inhibits epithelial-mesenchymal transition in glioblastoma cells by upregulating p53 and promoting miR-361-5p level to suppress ZEB1 expression
- PMID: 40597639
- PMCID: PMC12210478
- DOI: 10.1186/s12868-025-00956-6
Shikonin inhibits epithelial-mesenchymal transition in glioblastoma cells by upregulating p53 and promoting miR-361-5p level to suppress ZEB1 expression
Abstract
Objective: Shikonin, an active compound from the rhizome of Lithospermum erythrorhizon, exerts anti-tumor effects in various cancers, including glioblastoma multiforme (GBM). This study explored the mechanism of Shikonin for inhibiting the migration and invasion of GBM cells, providing a rationale for developing novel glioma therapies.
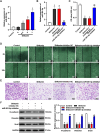
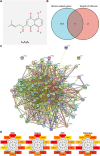
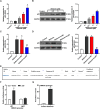
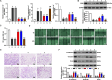
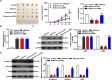
Methods: The effects of Shikonin on GBM cell proliferation, migration, invasion, and epithelial-mesenchymal transition (EMT) were detected by CCK-8, scratch wound-healing, Transwell, and Western blot assays. The effect of Shikonin on miR-361-5p expression in GBM cells was examined by RT-qPCR and the effect of miR-361-5p inhibitor transfection on proliferation, migration, invasion, and EMT in Shikonin-treated GBM cells was examined. Shikonin's target genes were identified and validated using dual luciferase reporter gene assay and chromatin immunoprecipitation (ChIP) assay, focusing on its induction of miR-361-5p expression. The downstream target genes of miR-361-5p were also identified and validated under Shikonin action. A GBM cell nude mouse xenograft tumor was established to confirm the regulatory role of Shikonin.
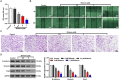
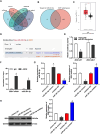
Results: Shikonin inhibited cell proliferation, migration, invasion, and EMT and upregulated miR-361-5p expression in GBM cells. Shikonin upregulated the glioma-associated protein p53, which promoted miR-361-5p transcription. miR-361-5p inhibited ZEB1 expression. Therefore, Shikonin inhibited GBM cell proliferation, migration, invasion, and EMT via p53/ miR-361-5p/ ZEB1 axis in vitro and in vivo.
Conclusion: Shikonin suppresses glioma cell proliferation, migration, invasion, and EMT by inhibiting ZEB1 expression through the p53/miR-361-5p axis.
Keywords: GBM; Shikonin; miR-361-5p.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The animal experiments reported in the manuscript were approved by the Animal Experimentation Ethics Committee of China Medical University, China. All experiments were performed in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines ( https://arriveguidelines.org ) for the reporting of animal experiments. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
The Antimicrobial Peptide Merecidin Inhibit the Metastasis of Triple-Negative Breast Cancer by Obstructing EMT via miR-30d-5p/Vimentin.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241281310. doi: 10.1177/15330338241281310. Technol Cancer Res Treat. 2024. PMID: 39267432 Free PMC article.
-
miR-429 suppresses progression and metastasis of salivary adenoid cystic carcinoma by targeting ZEB1.J Mol Histol. 2025 Jul 9;56(4):224. doi: 10.1007/s10735-025-10504-5. J Mol Histol. 2025. PMID: 40632321
-
RETRACTED: Shikonin Inhibits the Migration and Invasion of Human Glioblastoma Cells by Targeting Phosphorylated β-Catenin and Phosphorylated PI3K/Akt: A Potential Mechanism for the Anti-Glioma Efficacy of a Traditional Chinese Herbal Medicine.Int J Mol Sci. 2015 Oct 9;16(10):23823-48. doi: 10.3390/ijms161023823. Int J Mol Sci. 2015. Retraction in: Int J Mol Sci. 2025 Jun 20;26(13):5922. doi: 10.3390/ijms26135922. PMID: 26473829 Free PMC article. Retracted.
-
Molecular drivers of epithelial-mesenchymal transition (EMT) in glioblastoma and impact on therapy resistance.Pathol Res Pract. 2025 Aug;272:156111. doi: 10.1016/j.prp.2025.156111. Epub 2025 Jul 7. Pathol Res Pract. 2025. PMID: 40651122 Review.
-
The Epithelial-to-Mesenchymal Transition-Like Process in Glioblastoma: An Updated Systematic Review and In Silico Investigation.Med Res Rev. 2017 Mar;37(2):271-313. doi: 10.1002/med.21408. Epub 2016 Sep 12. Med Res Rev. 2017. PMID: 27617697
References
-
- Wesseling P, Capper D. WHO 2016 classification of gliomas. Neuropathol Appl Neurobiol. 2018;44(2):139–50. 10.1111/nan.12432. - PubMed
-
- Muir M, Gopakumar S, Traylor J, Lee S, Rao G. Glioblastoma multiforme: novel therapeutic targets. Expert Opin Ther Targets. 2020;24 7:605–14. 10.1080/14728222.2020.1762568. - PubMed
-
- Janjua TI, Rewatkar P, Ahmed-Cox A, Saeed I, Mansfeld FM, Kulshreshtha R, et al. Frontiers in the treatment of glioblastoma: past, present and emerging. Adv Drug Deliv Rev. 2021;171:108–38. 10.1016/j.addr.2021.01.012. - PubMed
-
- Hashmi FA, Salim A, Shamim MS, Bari ME. Biological characteristics and outcomes of gliosarcoma. J Pak Med Assoc. 2018;68 8:1273–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

