Infliximab for patients with moderate to severely active ulcerative colitis: an updated meta-analysis of randomized controlled trials
- PMID: 40597681
- PMCID: PMC12210827
- DOI: 10.1186/s12876-025-04065-w
Infliximab for patients with moderate to severely active ulcerative colitis: an updated meta-analysis of randomized controlled trials
Abstract
Background and aims: Ulcerative colitis (UC) is a serious inflammatory bowel disease with significant morbidity and mortality. Infliximab (IFX), a TNF-alpha antagonist, is recommended by the American Gastroenterological Association, but its clinical effectiveness and safety are based on limited studies. This meta-analysis of randomized controlled trials (RCTs) evaluates the efficacy and safety of IFX in moderate-to-severe active UC, providing evidence for its clinical application.
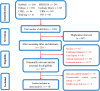
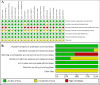
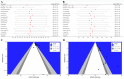
Methods: A systematic search of major English (PubMed, MEDLINE, Web of Science, Embase, Cochrane Library) and Chinese databases (CNKI, SinoMed, Wanfang, VIP) was conducted up to September 25, 2023, to identify RCTs comparing IFX against placebo, aminosalicylates, corticosteroids, or immunosuppressants for moderate-to-severe UC. Pooled analyses assessed short- and long-term clinical response, clinical remission, endoscopic remission (mucosal healing), safety outcomes, and corresponding 95% confidence intervals. Subgroup analyses, heterogeneity assessment, sensitivity analyses, publication bias evaluation (funnel plots), and meta-regression were performed.
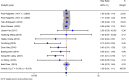
Results: Twenty RCTs involving 2,350 patients (IFX: n = 1,300; controls: n = 1,050) were included. IFX demonstrated significant superior efficacy versus controls across all primary endpoints: short-term (RR = 1.38, P < 0.001) and long-term clinical response (RR = 1.52, P = 0.007); short-term (RR = 1.38, P < 0.001) and long-term clinical remission (RR = 1.52, P = 0.022); short-term (RR = 1.58, P = 0.001) and long-term endoscopic remission (RR = 1.39, P = 0.010). Adverse event rates did not differ significant between groups (RR = 1.00, P = 0.933). Meta-regression suggested geographical region as a potential source of heterogeneity for short-term clinical response.
Conclusions: IFX has a more positive effect on active UC than placebos, aminosalicylic acid preparations, steroids, or immunosuppressive drugs, but shows no superiority in terms of adverse responses.
Keywords: Evidence-based practice; Infliximab; Meta-analysis; TNF-alpha antagonists; Ulcerative colitis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2012 Oct 17;10:CD000543. doi: 10.1002/14651858.CD000543.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Apr 21;4:CD000543. doi: 10.1002/14651858.CD000543.pub4. PMID: 23076889 Updated.
-
Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2016 Apr 21;4(4):CD000543. doi: 10.1002/14651858.CD000543.pub4. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Aug 12;8:CD000543. doi: 10.1002/14651858.CD000543.pub5. PMID: 27101467 Free PMC article. Updated.
-
Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (including a review of TA140 and TA262): clinical effectiveness systematic review and economic model.Health Technol Assess. 2016 May;20(39):1-326. doi: 10.3310/hta20390. Health Technol Assess. 2016. PMID: 27220829 Free PMC article.
References
-
- Shivananda S, Lennard-Jones J, Logan R, Fear N, Price A, Carpenter L, et al. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut. 1996;39(5):690–7. - PMC - PubMed
-
- Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6(1):74. - PubMed
-
- Gajendran M, Loganathan P, Jimenez G, Catinella AP, Ng N, Umapathy C, et al. A comprehensive review and update on ulcerative colitis(). Dis Mon. 2019;65(12):100851. - PubMed
-
- Olén O, Askling J, Sachs MC, Neovius M, Smedby KE, Ekbom A, et al. Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964–2014. Gut. 2020;69(3):453–61. - PubMed
-
- Zeng Z, Zhu Z, Yang Y, Ruan W, Peng X, Su Y, et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol. 2013;28(7):1148–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- No. 82260936/National Natural Science Foundation of China
- No. 82260936/National Natural Science Foundation of China
- No. 82260936/National Natural Science Foundation of China
- No. 82260936/National Natural Science Foundation of China
- No. 82260936/National Natural Science Foundation of China
- No. 82260936/National Natural Science Foundation of China
- No. 82260936/National Natural Science Foundation of China
- No. zyyzdxk-2023188/NATCMs Project of High-level Construction of Key TCM Disciplines
- No. zyyzdxk-2023188/NATCMs Project of High-level Construction of Key TCM Disciplines
- No. zyyzdxk-2023188/NATCMs Project of High-level Construction of Key TCM Disciplines
- No. zyyzdxk-2023188/NATCMs Project of High-level Construction of Key TCM Disciplines
- No. zyyzdxk-2023188/NATCMs Project of High-level Construction of Key TCM Disciplines
- No. zyyzdxk-2023188/NATCMs Project of High-level Construction of Key TCM Disciplines
- No. zyyzdxk-2023188/NATCMs Project of High-level Construction of Key TCM Disciplines
- GYZYYFY- BS-2022(02)/Funding for Ph.D. of Guizhou University of Traditional Chinese Medicine
- GYZYYFY- BS-2022(02)/Funding for Ph.D. of Guizhou University of Traditional Chinese Medicine
- GYZYYFY- BS-2022(02)/Funding for Ph.D. of Guizhou University of Traditional Chinese Medicine
- GYZYYFY- BS-2022(02)/Funding for Ph.D. of Guizhou University of Traditional Chinese Medicine
- GYZYYFY- BS-2022(02)/Funding for Ph.D. of Guizhou University of Traditional Chinese Medicine
- GYZYYFY- BS-2022(02)/Funding for Ph.D. of Guizhou University of Traditional Chinese Medicine
- GYZYYFY- BS-2022(02)/Funding for Ph.D. of Guizhou University of Traditional Chinese Medicine
LinkOut - more resources
Full Text Sources
Medical

