Prevalence of advanced liver fibrosis in the general population of the Paris region according to FIB-4 score and liver risk score
- PMID: 40597684
- PMCID: PMC12210717
- DOI: 10.1186/s12876-025-03992-y
Prevalence of advanced liver fibrosis in the general population of the Paris region according to FIB-4 score and liver risk score
Abstract
Background and aims: The ideal non-invasive marker to identify patient at risk for liver fibrosis in the general population is unknown. Current guidelines (EASL) recommend using the FIB-4 score as a screening tool but the low specificity may result in a large number of potentially false-positive results. Recently, the liver risk score (LRS) has been suggested to more accurately identify patients at-risk. The aim of our study was to identify populations at-risk using the two scores.
Materials and methods: “Cerbafib” was a prospective cohort derived from laboratories throughout the Paris region. The prevalence of advanced liver fibrosis was assessed using the non-invasive FIB-4 score with a cut-off of 2.67 (rule-in) and 1.3 (rule-out) and the LRS with a cut-off of < 6 (minimal risk), ≥ 6 to < 10 (low risk), ≥ 10 to < 15 (medium risk) and ≥ 15 (high risk).
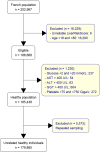
Results: Between January and April 2023, 179 865 patients were included in the cohort. The mean age was 52 years and 45% were men. FIB-4 identified 55 376 (31%) patients requiring specialist referral ( ≥ = 1.3) and 4002 patients (2.2%) with suspected advanced fibrosis. LRS identified 38 175 (21%) patients with estimated liver stiffness ≥ 6 kPa, 1 933 patients (1%) ≥ 10 kPa and 35 (0.02%) patients ≥ 15 kPa. There was a poor correlation between the two tests (r = 0.45).
Conclusion: The Liver Risk Score is a practical tool to identify a population in need of further hepatologic evaluation. Compared to FIB-4, the population identified by LRS is smaller and different. An adapted new pragmatic screening algorithm using LRS should be considered.
Keywords: Cirrhosis; Fibroscan (R); Liver-relates mortality; Non-invasive test; Screening.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the IRB ethics committee at Henri Mondor University Hospital (n° 00011558). All patients gave written informed consent for the use and the publication of their clinical data. The study was conducted in compliance with the Declaration of Helsinki. Consent for publication: NA. Competing interests: The authors declare no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

