CASCADE: a community-engaged action model for generating rapid, patient-engaged decisions in clinical research
- PMID: 40597746
- PMCID: PMC12211934
- DOI: 10.1186/s12874-025-02565-7
CASCADE: a community-engaged action model for generating rapid, patient-engaged decisions in clinical research
Abstract
Background: Integrating patient and community input is essential to the relevance and impact of patient-focused research. However, specific techniques for generating patient and community-informed research decisions remain limited. This manuscript describes a novel CASCADE method (Community-Engaged Approach for Scientific Collaborations and Decisions) that was developed and implemented to make actionable, patient-centered research decisions during a federally funded clinical trial.
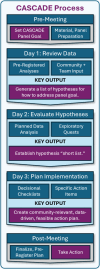
Methods: The CASCADE method was developed to facilitate decision-making, combining techniques from a variety of past methodologies with new approaches that aligned with project constraints and goals. The final result was a series of procedures that spanned seven thematic pillars (1) identifying a shared, specific, and actionable goal; (2) centering community input; (3) integrating both pre-registered statistical analyses and exploratory "quests"; (4) fixed-pace scheduling, supported by technology; (5) minimizing opportunities for cognitive biases typical to group decision making; (6) centering diversity experiences and perspectives, including those of individual patients; (7) making decisions that are community-relevant, rigorous, and feasible. The final approach was piloted within an active clinical trial, with the primary goal of describing feasibility (participation, discussion topics, timing, quantity of outputs).
Results: The inaugural CASCADE panel aimed to identify ways to improve an algorithm for matching patients to specific types of telehealth programs within an active, federally funded clinical trial. The panel was attended by 27 participants, including 5 community interest-holders. Data reviewed to generate hypotheses and make decisions included (1) pre-registered statistical analyses, (2) results of 12 "quests" that were launched during the panel to answer specific panelist questions via exploratory analyses or literature review, (3) qualitative and quantitative patient input, and (4) team member input, including by staff who represented the focal patient population for the clinical trial. CASCADE pillars were successfully integrated to generate 18 initial and 6 final hypotheses, which were translated to 19 decisional changes.
Conclusions: The CASCADE approach was an effective tool for rapidly, efficiently making patient-centered decisions during an ongoing, federally funded clinical trial. Opportunities for further development will include exploring best-practice structural procedures, enhancing greater opportunities for pre-panel input by community interest-holders, and determining how to best standardize CASCADE outputs.
Trial registration: The CASCADE procedure was developed in the context of NCT05999448.
Keywords: CASCADE; Clinical trials; Community-based participatory research; Decision making; Delphi panel; Patient acceptability; Patient engagement; Project wellcast.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: No human subjects research is directly reported in this manuscript. The broader human subjects protocol for the affiliated project, Project WellCAST, was approved by Purdue University IRB #2022 − 1580. All participants provided informed consent. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Update of
-
CASCADE: A Community-Engaged Action Model for Generating Rapid, Patient-Engaged Decisions in Clinical Research.Res Sq [Preprint]. 2024 Aug 27:rs.3.rs-4790564. doi: 10.21203/rs.3.rs-4790564/v1. Res Sq. 2024. Update in: BMC Med Res Methodol. 2025 Jul 1;25(1):168. doi: 10.1186/s12874-025-02565-7. PMID: 39257986 Free PMC article. Updated. Preprint.
References
-
- Graham KL, Green S, Kurlan R, Pelosi JS. A Patient-Led Educational Program on Tourette Syndrome: Impact and Implications for Patient-Centered Medical Education. Teach Learn Med. 2014;26(1):34–9. Available from: 10.1080/10401334.2013.857339 - PubMed
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous