Inhibition of Axl attenuates acute kidney injury by alleviating inflammation via SOCS3 downregulation in tubular epithelial cells
- PMID: 40597777
- PMCID: PMC12220096
- DOI: 10.1186/s12882-025-04222-z
Inhibition of Axl attenuates acute kidney injury by alleviating inflammation via SOCS3 downregulation in tubular epithelial cells
Abstract
Background: In acute kidney injury (AKI), inflammatory crosstalk between tubular epithelial cells (TECs) and immune cells drives disease progression. Although the Axl-SOCS3 axis in myeloid cells typically suppresses inflammation, TEC-specific SOCS3 deletion paradoxically protects against AKI, suggesting a cell type-specific pro-inflammatory role.
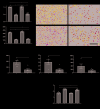
Methods: We induced AKI via bilateral ischemia/reperfusion (IRI) in mice. The Axl-specific pharmacological inhibitor R428 was administered via subcutaneous injection immediately post-IRI, with plasma and kidney samples collected 24 h later. To assess the effects of SOCS3 in TECs, small interfering RNA was used to silence SOCS3 in cisplatin injured HK2 cells. Axl/SOCS3 expression levels were assessed in human AKI biopsies.
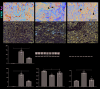
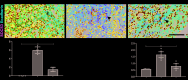
Results: In AKI patients and IRI mice, Axl was upregulated in interstitial immune cells, while SOCS3 increased in TECs. Axl inhibition by R428 attenuated renal injury, reducing inflammatory infiltration, NF-κB p65 phosphorylation, and TEC SOCS3 expression. Notably, SOCS3 knockdown in TECs suppressed NF-κB activation and IL-1β/IL-6 production, implicating Axl-SOCS3 as a pro-inflammatory amplifier in AKI.
Conclusion: The Axl-SOCS3 axis exacerbates AKI by reinforcing NF-κB-driven inflammation in TECs, creating a vicious cycle between immune cells and TECs. Targeting this cross-cellular pro-inflammatory pathway offers a promising therapeutic strategy for AKI.
Keywords: Acute kidney injury; Axl; Inflammation; Suppressor of cytokine signaling 3; Tubular epithelial cells.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were approved by the Institutional Animal Care and Use Committee of Tianjin Medical University General Hospital (Approval Number: IRB2022-ZWFL-070). All patients provided written informed consent. The clinical protocols were performed in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of Tianjin Medical University General Hospital (Approval Number: IRB2020-YX-114-01). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1949–64. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

