The complex journey of targeting RAS in oncology
- PMID: 40597785
- PMCID: PMC12211582
- DOI: 10.1186/s12885-025-14033-y
The complex journey of targeting RAS in oncology
Abstract
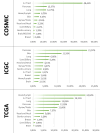
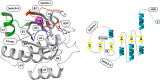
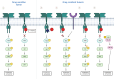
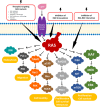
Given the prevalence of RAS mutations in various cancers, personalized therapeutic approaches, guided by molecular markers, are essential. Farnesyltransferase inhibitors (FTIs) have emerged as potential therapeutic options; however, they also face obstacles such as toxicity and limited efficacy. Alternative strategies, such as direct inhibitors combined with pathway modulators, RNA interference, and gene-editing technologies, are under clinical investigation. The targeting of RAS, complicated by its structural nuances, particularly in the G domain, has advanced with the identification of druggable pockets such as the SW-II pocket. This breakthrough has led to the development of targeted therapeutics, such as sotorasib and adagrasib, for KRAS G12C-mutated non-small cell lung cancer (NSCLC). However, these advancements face challenges, including adaptive resistance and the necessity for isoform selectivity. New inhibitors, such as LY3537982 or GDC-6036, are promising, but achieving effective and selective RAS inhibition remains a significant challenge. Additionally, clinical trials have highlighted variability in patient responses, attributing limited treatment efficacy to resistance mechanisms, including on-target mutations and off-target pathway activations. Finally, the RAS oncogene, traditionally viewed as predominantly pro-cancerous, plays a complex role in oncogenesis, with recent evidence suggesting context-dependent effects, such as inducing senescence in certain cells. This shift in understanding underscores the therapeutic potential of manipulating the interplay between RAS and TP53 mutations in cancer. In conclusion, the complexity of effectively targeting the RAS-RAF-ERK pathway is exacerbated by the diverse resistance mechanisms. Challenges such as off-target effects and delivery issues remain significant barriers in the introduction of effective therapies based on RAS inhibitors. This overview highlights the evolving nature of targeting RAS in cancer therapy.
Keywords: Cancer; Drug discovery; HRAS; KRAS; NRAS; Oncogene; Protein structure; RAS proteins; Senescence; Therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

