Efficacy and safety of finerenone in non-diabetic CKD patients: a single-center, real-world, retrospective study
- PMID: 40597854
- PMCID: PMC12218821
- DOI: 10.1186/s12882-025-04241-w
Efficacy and safety of finerenone in non-diabetic CKD patients: a single-center, real-world, retrospective study
Abstract
Background: Finerenone, a novel non-steroidal mineralocorticoid receptor antagonist, has shown promising efficacy and safety profiles in the management of chronic kidney disease (CKD) associated with type 2 diabetes mellitus (T2DM). However, evidence for its role in non-diabetic CKD patients require further investigation.
Methods: This retrospective, real-world study involved non-diabetic CKD patients from April 2023 to June 2024. Participants received finerenone alongside standard CKD treatment. Primary clinical results included changes in the urinary albumin-to-creatinine ratio (UACR), estimated glomerular filtration rate (eGFR), and serum potassium (sK+) levels. The data were collected initially and during follow-ups at 1, 3, 6, and 12 months.
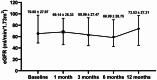
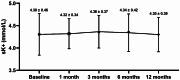
Results: In total, 37 non-diabetic CKD patients were included in the population; 21 individuals (56.8%) were male, and the mean age was 48.84 ± 14.69 years. During the follow-up, there was a notable decrease in UACR, with a median reduction of 664.95 mg/g (IQR, 196.60-1226.70, P = 0.002). The baseline average eGFR was 70.80 ± 27.97 mL/min/1.73m2, with no notable alterations observed during the follow-up (P > 0.05). In terms of safety analysis, the sK + levels were within the 3.5-5.5 mmol/L range, with no significant difference from the baseline (P > 0.05). No patients discontinued treatment or were hospitalized because of hyperkalemia.
Conclusion: Real-world practice indicates that finerenone is effective and safe for non-diabetic CKD patients, but further large-scale, prospective studies are needed to confirm these findings.
Keywords: Chronic kidney disease; Finerenone; Non-diabetic.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethics Committee of Zhongshan Hospital of Xiamen University (IRB approval No. 2025-026), with the requirement for informed consent waived in accordance with the retrospective observational study design. Ethics approval was applied in accordance with the Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Change in Urine Albumin-Creatinine Ratio and Occurrence of Hyperkalemia in Patients Initiating Finerenone in the USA: A Cohort Study from the FOUNTAIN Platform.Nephron. 2025;149(7):371-383. doi: 10.1159/000543923. Epub 2025 Feb 27. Nephron. 2025. PMID: 40015260 Free PMC article.
-
COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using a UACR Endpoint (CONFIDENCE) trial: baseline clinical characteristics.Nephrol Dial Transplant. 2025 Aug 1;40(8):1559-1569. doi: 10.1093/ndt/gfaf022. Nephrol Dial Transplant. 2025. PMID: 39916475 Free PMC article. Clinical Trial.
-
Observation of the therapeutic effect of finerenone, a novel non-steroidal mineralocorticoid receptor antagonist, in patients with non-diabetic CKD.Ren Fail. 2025 Dec;47(1):2545939. doi: 10.1080/0886022X.2025.2545939. Epub 2025 Aug 12. Ren Fail. 2025. PMID: 40797285 Free PMC article.
-
Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis.Health Technol Assess. 2010 Apr;14(21):1-184. doi: 10.3310/hta14210. Health Technol Assess. 2010. PMID: 20441712
-
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1 to 3) non-diabetic chronic kidney disease.Cochrane Database Syst Rev. 2023 Jul 19;7(7):CD007751. doi: 10.1002/14651858.CD007751.pub3. Cochrane Database Syst Rev. 2023. PMID: 37466151 Free PMC article.
References
-
- Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease Lancet. 2017;389(10075):1238–52. - PubMed
-
- Barrera-Chimal J, Girerd S, Jaisser F. Mineralocorticoid receptor antagonists and kidney diseases: pathophysiological basis. Kidney Int. 2019;96(2):302–19. - PubMed
-
- Epstein M. Aldosterone and mineralocorticoid receptor signaling as determinants of cardiovascular and renal injury: from Hans Selye to the present. Am J Nephrol. 2021;52(3):209–16. - PubMed
-
- Patel V, Joharapurkar A, Jain M. Role of mineralocorticoid receptor antagonists in kidney diseases. Drug Dev Res. 2021;82(3):341–63. - PubMed
-
- Barrera-Chimal J, Lima-Posada I, Bakris GL, Jaisser F. Mineralocorticoid receptor antagonists in diabetic kidney disease - mechanistic and therapeutic effects. Nat Rev Nephrol. 2022;18(1):56–70. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

