Efficacy and safety of first-line chemoimmunotherapy in young patients with extensive-stage small cell lung cancer: a multicenter retrospective study
- PMID: 40597860
- PMCID: PMC12220248
- DOI: 10.1186/s12885-025-14524-y
Efficacy and safety of first-line chemoimmunotherapy in young patients with extensive-stage small cell lung cancer: a multicenter retrospective study
Abstract
Objectives: Chemoimmunotherapy is the first-line treatment for extensive-stage small cell lung cancer (ES-SCLC). This study aims to evaluate the survival outcomes and safety of chemoimmunotherapy in young patients with ES-SCLC.
Patients and methods: Patients with pathologically or cytologically confirmed ES-SCLC from three centers and divided into two age groups: young (aged ≤ 45 years) and control (aged > 45 to ≤ 75 years) between January 2015 and December 2023. We assessed progression-free survival (PFS), overall survival (OS), and safety between the two age groups.
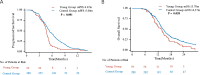
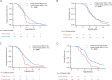
Results: Of the whole 347 patients, 59 were in the young group, while 288 were in the control group. The young group exhibited poorer PFS (median, 4.67 vs. 5.40 months, p < 0.001) and OS (median, 13.7 vs. 14.4 months, p = 0.028) compared with the control group, particularly in the context of chemoimmunotherapy [PFS (median, 4.50 vs. 5.57 months; p = 0.002), OS (median, 13.20 vs. 15.33 months; p = 0.012), respectively]. Additionally, in the young group, chemoimmunotherapy showed similar PFS (median, 4.50 vs. 5.75 months; p = 0.501) and OS (median, 13.20 vs. 13.70 months; p = 0.508) compared to chemotherapy. Moreover, the young group had a higher incidence of immune-related adverse events (irAEs) (30.51% vs. 11.46%, p < 0.001) and hematologic toxicity, including thrombocytopenia (25.42% vs. 14.24%, p = 0.033).
Conclusions: The young group had poorer survival outcomes and chemoimmunotherapy may not provide a survival benefit in young patients, as evidenced by similar PFS and OS compared to chemotherapy. Additionally, the young group also experienced a higher incidence of immune-related adverse events (irAEs) and hematologic toxicity.
Keywords: Adults; Chemoimmunotherapy; Drug-Related side effects and adverse reactions; Extensive-stage small cell lung cancer; Older adults.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by Ethics Committee of PLA General Hospital and was conducted strictly according to the principles of the Declaration of Helsinki. The need for informed consent was waived by Ethics Committee of PLA General Hospital because of the retrospective nature of the study and the use of anonymized data for analysis. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Consolidative thoracic radiotherapy improves the prognosis of extensive stage small-cell lung cancer patients in the chemoimmunotherapy era: a multicenter retrospective analysis.Ann Med. 2025 Dec;57(1):2542434. doi: 10.1080/07853890.2025.2542434. Epub 2025 Aug 4. Ann Med. 2025. PMID: 40755353 Free PMC article.
-
Toripalimab Plus Chemotherapy as a First-Line Therapy for Extensive-Stage Small Cell Lung Cancer: The Phase 3 EXTENTORCH Randomized Clinical Trial.JAMA Oncol. 2025 Jan 1;11(1):16-25. doi: 10.1001/jamaoncol.2024.5019. JAMA Oncol. 2025. PMID: 39541202 Clinical Trial.
-
Survival impact of immune-related adverse events in extensive stage small cell lung cancer patients: a retrospective cohort study.Immunotherapy. 2025 Jan;17(1):19-24. doi: 10.1080/1750743X.2025.2456448. Epub 2025 Jan 23. Immunotherapy. 2025. PMID: 39844686
-
Platinum versus non-platinum chemotherapy regimens for small cell lung cancer.Cochrane Database Syst Rev. 2015 Aug 2;2015(8):CD006849. doi: 10.1002/14651858.CD006849.pub3. Cochrane Database Syst Rev. 2015. PMID: 26233609 Free PMC article.
-
Efficacy and safety of first-line immunotherapy plus chemotherapy in treating patients with extensive-stage small cell lung cancer: a Bayesian network meta-analysis.Front Immunol. 2023 Jun 26;14:1197044. doi: 10.3389/fimmu.2023.1197044. eCollection 2023. Front Immunol. 2023. PMID: 37435087 Free PMC article.
References
-
- van Meerbeeck JP, et al. Small-cell lung cancer. Lancet (London England). 2011;378(9804):1741–55. - PubMed
-
- Siegel RL, et al. Cancer Stat 2021 CA: cancer J Clin. 2021;71(1):7–33. - PubMed
-
- Sung H, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Horn L, et al. First-Line Atezolizumab plus chemotherapy in Extensive-Stage Small-Cell lung Cancer. N Engl J Med. 2018;379(23):2220–9. - PubMed
-
- Paz-Ares L, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet (London England). 2019;394(10212):1929–39. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

